Cimetidine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Cimetidine | ||||||||||||
| other names |
2-cyano-1-methyl-3- [2- (5-methylimidazol-4-ylmethylsulfanyl) ethyl] guanidine ( IUPAC ) |
||||||||||||
| Molecular formula | C 10 H 16 N 6 S | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class |
Gastric acid blocker , immunostimulant |
||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | 252.34 g mol −1 | ||||||||||||
| Melting point |
|
||||||||||||
| pK s value |
6.8 |
||||||||||||
| solubility |
slightly soluble in water (9.38 g l −1 at 25 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cimetidine is a drug that is used as an H 2 antihistamine to suppress gastric juice production. Chemically, the substance belongs to the class of drugs guanidine - derivatives .
development
Cimetidine is the first H 2 antagonist to be introduced in the treatment of heartburn and gastrointestinal ulcers . It was developed by SmithKline and French (now GlaxoSmithKline ) from the mid-1960s and came onto the market in 1976 as Tagamet . Tagamet became one of the first blockbusters in the pharmaceutical market.
The British chemists Graham J. Durant (* 1934), John Colin Emmett (* 1939) and C. Robin Ganellin (* 1934), who were inducted into the National Inventors Hall of Fame , are considered to be the developers.
Extraction and presentation
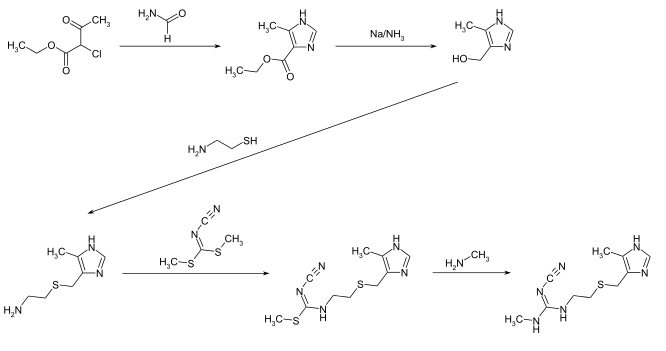
The compound can be produced in a five-step synthesis. In the first step, the imidazole structural element is formed through the reaction of ethyl 2-chloro-3-oxobutyrate with formamide . The subsequent reduction with sodium in liquid ammonia gives the intermediate 4-hydroxymethyl-5-methylimidazole. This is followed by the reactions with 2-aminoethanethiol and dimethylcyanocarboimidodithionate. The latter compound can be obtained from cyanamide , carbon disulfide and dimethyl sulfate in the presence of potassium hydroxide solution . In the last synthesis step, the methylthio group is substituted by the methylamino group using methylamine.
properties
Cimetidine occurs in four polymorphic forms and one monohydrate form. The melting points for polymorph A are 140.3 ° C, for polymorph B 142.0 ° C and polymorphs C and D each 141.9 ° C. The dehydration of the monohydrate leads to the polymorph A. The crystal structures of the individual polymorphs were determined by means of X-ray diffraction . The different crystal forms differ in their bioavailability based on a different dissolution behavior . Only forms A and B are used in pharmaceutical formulations.
pharmacology
Mechanism of action
Cimetidine is a competitive, reversible H 2 antagonist of the parietal cells of the gastric mucosa . Thus, it reduces the secretion of stomach acid and the release of the digestive enzyme pepsin , but does not affect the formation of stomach mucus and gastric emptying. In addition, the vagus and gastrin-induced acid release is not yet competently suppressed.
In addition, cimetidine inhibits the H 2 receptors on T suppressor cells and prevents their action, which leads to indirect immune stimulation. It also reduces the secretion of parathyroid hormone and androgens .
More recent guidelines, however, prefer proton pump inhibitors to H2 blockers in the treatment of gastritis, esophagitis and reflux diseases.
Distribution in the body
Cimetidine is administered orally or parenterally and around 70% is absorbed in the intestine, mainly in the ileum . It is distributed in the bloodstream and also gets into the milk and crosses the placenta . It is metabolized in the liver and some of it is excreted unchanged in the urine . The half-life is about an hour.
application areas
- Ulcers in the stomach, abomasum, and duodenum
- Acute pancreatitis and exocrine pancreatic insufficiency
- gastritis
- Esophagitis and gastroesophageal reflux
- Duodenal gastric reflux
- Bleeding in the upper gastrointestinal tract
- Gastrinomas and systemic mastocytosis
- Immune stimulation, use e.g. B. in melanoma in horses
Contraindications and side effects
Use is contraindicated in case of hypersensitivity and severe kidney or liver disease. Use on food-producing animals is not permitted.
Mental confusion, headache, gynecomastia , decreased libido , cardiac arrhythmias ( bradycardia ), and rarely agranulocytosis have been observed in humans . It also has a high delirium- inducing potency. There are no reports of side effects in veterinary medicine.
Interactions
Since cimetidine has an inhibitory effect on some cytochrome P450 enzymes, numerous interactions are possible through prolongation and intensification of the effect and thus leads to incompatibility with drugs that act as enzyme inducers on CYP-450 enzymes or are degraded by them become. This is also one of the reasons why cimetidine is not available for over-the-counter OTC sales compared to the other H 2 blockers like ranitidine and famotidine . Furthermore, cimetidine is no longer the therapy recommendation of first choice because ranitidine (and proton pump inhibitors ) is a longer and more potent drug with better tolerability with regard to gastric acid inhibition.
Cimetidine inhibits creatinine secretion in the kidneys and can therefore, in laboratory tests, simulate a reduced glomerular filtration rate . The main reason for this is that cimetidine drains the efflux from the proximal tubular cells via the two SLC transporters MATE1 (SLC47A1, multidrug and toxin extrusion 1 ) and MATE2-K (SLC47A2, multidrug and toxin extrusion 2 - kidney ) on the apical side inhibited. In addition, cimetidine - albeit somewhat weaker than MATE1 and MATE2-K - also inhibits the organic cation transporter 2 (OCT2) on the basolateral side.
Trade names
Cimetag (A), CimLich (D), H 2 Blocker (D), Neutromed (A), Ulcostad (A) and other generics (D, A)
Web links
- Entry on cimetidine at Vetpharm, accessed on August 11, 2012.
Individual evidence
- ↑ a b c d e Calvo, NL; Maggio, RM; Kaufman, TS: A dynamic thermal ATR-FTIR / chemometric approach to the analysis of polymorphic interconversions. Cimetidine as a model drug In: J Pharm Biomed Anal . 92 (2014) 90-97, doi : 10.1016 / j.jpba.2013.12.036
- ↑ a b Entry on cimetidine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet cimetidine from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ↑ a b c d e f g A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2000), Thieme-Verlag Stuttgart, ISBN 978-1- 58890-031-9 .
- ↑ a b c d e f g Entry on cimetidine. In: Römpp Online . Georg Thieme Verlag, accessed on July 12, 2014.
- ↑ The history of GSK . glaxosmithkline.de. Retrieved July 5, 2012.
- ↑ K. Harsányi, G. Domány, L. Toldy: Cimetidine monohydrate and processes for its preparation and use , Patent GB 2101991, June 24, 1982.
- ↑ M. Shibata, H. Kokubo, K. Morimoto, K. Morisaka, T. Ishida, M. Inoue: X-ray structural studies and physicochemical properties of cimetidine polymorphism . In: J Pharm Sci . 72 (1983) 1436-1442, doi : 10.1002 / jps.2600721217 .
- ↑ DA Middleton, CSL Duff, X. Peng, DG Reid, D. Saunders: Molecular conformations of the polymorphic forms of cimetidine from 13 C solid-state NMR distance and angle measurements . In: J. Am. Chem. Soc. 122 (2000) 1161-1170, doi : 10.1021 / ja993067z .
- ↑ E. Hadicke, F. Frickel, A. Franke: The structure of cimetidine (N-cyano-N-methyl-N '- [2 - [[(5-methyl-1H-imidazol-4-yl) methyl] thio ] ethyl] guanidine), a histamine H2 receptor antagonist . In Chem. Ber. 111 (1978) 3222-3232, doi : 10.1002 / cber.19781110926 .
- ↑ L. Párkányi, A. Kálmán, B. Hegedüs, K. Harsanyi, J. Kreidl: Structure of a novel and reproducible polymorph (Z) of the histamine H2-receptor antagonist cimetidine, C 10 H 16 N 6 S . In: Acta Cryst. C40 (1984) 676-679.
- ↑ A. Arakcheeva, P. Pattison, A. Bauer-Brandl, H. Birkedal, G. Chapuis: Cimetidine C 10 H 16 N 6 S, form C: crystal structure and modeling of polytypes using the superspace approach . In: J. Appl. Cryst. 46 (2013) 99-107.
- ↑ H. Kokubo, K. Morimoto, T. Ishida, M. Inoue, K. Morisaka: Bioavailability and inhibitory effect for stress ulcer of cimetidine polymorphs in rats . In: Int J Pharm . 35 (1987) 181-183, doi : 10.1016 / 0378-5173 (87) 90088-3 .
- ↑ T. Funaki, S. Furata, N. Kaneniwa: Discontinuous absorption property of cimetidine . In: Int J Pharm . 31 (1986) 119-123, doi : 10.1016 / 0378-5173 (86) 90220-6 .
- ↑ H. Kamiya, K. Morimoto, K. Morisaka: Dissolution behavior and bioavailability of cimetidine – HCl (cimetidine monohydrochloride monohydrate) . In: Int J Pharm . 26 (1985) 197-200, doi : 10.1016 / 0378-5173 (85) 90212-1 .
- Jump up ↑ GHW Sanders, CJR Roberts, A. Danesh, AJ Murray, DM Price, MC Davies, SJB Tendler, MJ Wilkins: Discrimination of polymorphic forms of a drug product by localized thermal analysis . In: J. Microsc . 198 (2000) 77-81, doi : 10.1046 / j.1365-2818.2000.00709.x .
- ↑ a b Mutschler, Geisslinger, Kroemer, Ruth, Schäfer-Korting, Mutschler drug effects, 9th edition, 2008, ISBN 3-8047-1952-X .
- ↑ Schmidmaier R .: Influence of the regulatory function of mononuclear cells of the peripheral blood (PBMC) in vitro. Dissertation, Munich 2001. Accessed November 1, 2012 . (PDF).
- ↑ C. Gordon: Differential diagnosis of cimetidine-induced delirium. In: Psychosomatics. Volume 22, Number 3, March 1981, pp. 251-252, doi : 10.1016 / S0033-3182 (81) 73536-9 . PMID 7220787 .
- ↑ WW Weddington, AE Muelling a. a .: Cimetidine toxic reactions masquerading as delirium tremens. In: JAMA. Volume 245, Number 10, March 1981, pp. 1058-1059, PMID 7463626 .
- ^ A. Strauss: Cimetidine and delirium: Assessment and management. In: Psychosomatics. Volume 23, Number 1, January 1982, pp. 57-62, doi : 10.1016 / S0033-3182 (82) 70811-4 . PMID 7058250 .
- ^ Ruß, Endres, Arzneimittelpocket Plus 2008, 4th edition Oct. 2007, ISBN 978-3-89862-287-5 .
- ^ Lesley A. Stevens, Shani Shastri, Andrew S. Levery: Assessment of Renal Function in Jürgen Floege, Richard J Johnson, John Feehally: Comprehensive Clinical Nephrology , 4th Edition, St. Louis, 2010 pp. 32f.
- ↑ Michael Freissmuth: Pharmacokinetics. In: Michael Freissmuth, Stefan Offermanns, Stefan Böhm: Pharmacology and Toxicology. Springer, 2012, ISBN 3-642-12353-8 , p. 27. Limited preview in the Google book search.
- ^ S. Ito, H. Kusuhara et al. a .: Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. In: Journal of Pharmacology and Experimental Therapeutics . Volume 340, Number 2, February 2012, pp. 393-403, doi : 10.1124 / jpet.111.184986 . PMID 22072731 .
- ↑ H. Ehrsson, I. Wallin et al. a .: Cimetidine as an organic cation transporter antagonist. In: The American Journal of Pathology . Volume 177, number 3, September 2010, pp. 1573-1574, doi : 10.2353 / ajpath.2010.100484 . PMID 20651231 . PMC 2928986 (free full text).
- ↑ Red List, 08/2009.
- ↑ AM comp. d. Switzerland, 08/2009.
- ↑ AGES-PharmMed, 08/2009.