Amifostine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
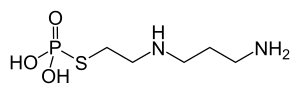
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Amifostine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 15 N 2 O 3 PS | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 214.22 g · mol -1 | ||||||||||||||||||
| Melting point |
Decomposes at 160–161 ° C (as monohydrate) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Amifostine is the international non-proprietary name for a cytoprotective effective drug .
description
Amifostine is a phosphorylated aminothioalcohol. As a prodrug amifostine is membrane-bound alkaline phosphatases of the endothelial cells into the actual active compound 2 - decomposes ((Aminopropyl) amino) ethanethiol (development code WR-1065). Amifostine itself is inactive.
In a large number of preclinical studies , a chemo- and radioprotective effect could be demonstrated on model organisms such as mice, dogs and monkeys . In clinical studies , supportive efficacy was also found in established therapy concepts.
Amifostine is the first and so far (as of 2010) only approved radio protector. Amifostine has been approved for kidney, blood and nerve protection by intravenous administration in Germany and the United States since 1995. In 1999 the approval for the treatment and prevention of dry mouth was extended.
Mechanism of action
| Organ or system | Protection factor |
|---|---|
| Bone marrow | 2.4-3.0 |
| immune system | 1.8-3.4 |
| skin | 2.0-2.4 |
| Small intestine | 1.8-2.0 |
| Colon | 1.8 |
| lung | 1.2-1.8 |
| esophagus | 1.4 |
| kidney | 1.5 |
| liver | 2.7 |
| Salivary gland | 2.0 |
| Oral mucosa | > 1 |
| Testicles | 2.1 |
The cell-protecting (cytoprotective) effect of amifostine, or of its metabolic product WR-1065, is based on the interception of free radicals and reactive oxygen species (ROS), the protection of the DNA , the acceleration of DNA repair and the induction of cellular oxygen deficiency ( hypoxia ) . The latter reduces the formation of free radicals and reactive oxygen species.
Amifostine works selectively in healthy cells. In all previous studies, no protection was observed a tumor - malignant ( malignant ) tissue will not benefit from the effect of amifostine. There are several reasons for this. One of them is the poor blood supply (hypovascularization) of many tumors, which restricts the transport of the active ingredient to the tumor - compared to healthy tissue. The low pH value in the tumor and its immediate surroundings - caused by tissue acidosis caused by anaerobic glycolysis ( Warburg effect ) - also contributes to the cytoprotective selectivity. In addition, the alkaline phosphatases are expressed much less strongly in the tumor tissue . Of the acid phosphatases that predominate in tumors , amifostine is not dephosphorylated.
If WR-1065 has been absorbed by the cells as an active substance, it can protect the DNA in the cell nucleus from damage or promote the repair mechanisms by scavenging free radicals, reducing the oxygen content in the cell and reacting and inactivating with cytotoxic agents.
In addition to these effects, other protective effects of amifostine were found. It stimulates the hematopoietic stem cells , modifies gene expression and enzyme activities , and shows inhibition of apoptosis . Amifostine may also protect against the development of secondary, therapy-related tumors. In vivo , an increase in the resistance of healthy cells to ionizing radiation and to alkylating agents such as nitrogen mustard derivatives , cyclophosphamide or melphalan , and other cytostatics such as cisplatin , anthracyclines and taxanes could be measured up to threefold.
Pharmacokinetics
The plasma half-life of amifostine is approximately eight minutes; the distribution half-life at about 0.8 minutes. Only about 4% of amifostine is bound to plasma proteins.
The short plasma half-life results primarily from the rapid metabolism in WR-1065, which itself has a plasma half-life of approximately 11 minutes. WR-1065 is quickly taken up by the cells or further metabolized into a disulfide (WR-33278). Amifostine is not available orally.
In preclinical studies, it was found that amifostine can selectively protect almost all healthy tissues from the cytotoxic effects of some chemotherapy drugs, as well as radiation therapy. The enrichment factor of WR-1065 in healthy tissue compared to malignant tissue is around 100: 1. It accumulates mainly in the bone marrow , salivary gland , kidneys and heart , as well as in the liver and the small intestinal mucosa . In animal models, peak tissue concentrations are reached within 5 to 15 minutes. Only small amounts of amifostine, between 1 and 4% of the amount injected, are excreted in the urine.
Amifostine cannot cross the blood-brain barrier . The central nervous system , which is dose-limiting in radiotherapy in many cases , is therefore not protected by amifostine.
application
Amifostine is administered intravenously , usually as a short infusion. The usual dose before chemotherapy is 740 to 900 mg / m² body surface area and before radiotherapy 250 to 350 mg / m². This amount of active ingredient is usually administered as a 15-minute short infusion about half an hour before a radiation session.
Since 1995 it has been approved in Germany and the United States for kidney, blood and nerve protection by means of intravenous administration. It has also been approved for the treatment and prevention of dry mouth since 1999. Amifostine is the first approved radio protector.
The preventive application of amifostine in the space , as a radioprotectant against the by solar flares ( solar particle events caused SPE) radiation exposures is discussed.
In the model organism color rat , nephropathies caused by ionizing radiation could be significantly reduced by inhibiting oxidative stress .
Side effects
Serious very common side effects after the infusion of amifostine include arterial hypotension . In a study with high dose amifostine (910 mg / m²) in ovarian cancer , transient hypotension was observed in 62% of patients. Hypotension began a mean of 14 minutes after the start of the 15-minute infusion and lasted an average of 6 minutes. In some cases this resulted in the infusion being stopped. In all cases, blood pressure returned to normal after 5 to 15 minutes. Less than 3% of patients discontinued supportive treatment with amifostine because of this side effect. In a study with patients with head and neck cancer , the amifostine dose was 200 mg / m² given before radiation therapy. In this study, hypotension was observed in 15% of patients.
Nausea and vomiting are also among the more common side effects, affecting around 10% of patients. Hypocalcaemia is occasionally seen in about 1% of all patients. Rare are the best as an expression of drug allergy adverse reactions such as Stevens-Johnson syndrome , Lyell's syndrome (toxic epidermal necrolysis), erythroderma as a result of a drug eruption , fever , chills , sneezing , somnolence and hiccups and anaphylaxis . Consciousness is rarely lost.
Contraindications (contraindications)
Amifostine should not be given to patients with arterial hypotension , or to patients with allergic reactions to amifostine.
Development history
In 1948 a working group led by the American radiologist Harvey Milton Patt (1918–1982) made the discovery that the amino acid cysteine has a radioprotective effect. This was the basis for the development of WR-2721. Later it was theorized that the mechanism of action is essentially determined by the thiol group, which can bind both free radicals and various alkylating agents. WR-2721 was developed at the Walter Reed Military Hospital in the late 1950s as part of a secret research project by the US Army ( US Army Anti-Radiation Drug Development Program ). Hence the code WR-2721 for Walter Reed 2721 . The aim of the project was to develop a radio protector to protect soldiers in a nuclear war . In a screening of over 4400 substances tested, WR-2721 showed the highest radioprotective effect, with a simultaneously high therapeutic range and good tolerability. After its development, the WR-2721 was not used for its original purpose. The main reason for this was that WR-2721 is not effective orally and therefore should have been administered intravenously by the soldiers themselves.
Finished medicinal products
Ethyol ( D )
literature
- Amifostine for cytoprotection against side effects of combination therapy with cyclophosphamide and cisplatin in patients with ovarian cancer. In: Der Arzneimittelbrief , Volume 31, 1997, 6b
- FA Mettler, D. Brenner, CN Coleman, JM Kaminski, AR Kennedy, LK Wagner: Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants. In: AJR. American Journal of Roentgenology , Volume 196, Number 3, March 2011, pp. 616-618, doi: 10.2214 / AJR.10.4959 . PMID 21343505 . (Review).
- LG Marcu: The role of amifostine in the treatment of head and neck cancer with cisplatin-radiotherapy. In: European Journal of Cancer Care , Volume 18, Number 2, March 2009, pp. 116-123, doi: 10.1111 / j.1365-2354.2008.01032.x . PMID 19267726 . (Review).
- LK Mell, B. Movsas: Pharmacologic normal tissue protection in clinical radiation oncology: focus on amifostine. In: Expert Opinion on Drug Metabolism & Toxicology , Volume 4, Number 10, October 2008, pp. 1341-1350, doi: 10.1517 / 17425255.4.10.1341 . PMID 18798703 . (Review).
- NP Praetorius, TK Mandal: Alternate delivery route for amifostine as a radio- / chemo-protecting agent. In: Journal of Pharmacy and Pharmacology , Volume 60, Number 7, July 2008, pp. 809-815, doi: 10.1211 / jpp.60.7.0001 . PMID 18549666 . (Review).
- MI Koukourakis, E. Maltezos: Amifostine administration during radiotherapy for cancer patients with genetic, autoimmune, metabolic and other diseases. In: Anti-Cancer Drugs Volume 17, Number 2, February 2006, pp. 133-138, PMID 16428930 . (Review).
- AC Müller: Value of the Comet Assay for the detection of radio protection. (PDF; 1.6 MB) Dissertation, Martin Luther University Halle-Wittenberg, 2003
- V. Santini: Amifostine: chemotherapeutic and radiotherapeutic protective effects. In: Expert Opinion on Pharmacotherapy , Volume 2, Number 3, March 2001, pp. 479-489, doi: 10.1517 / 14656566.2.3.479 . PMID 11336600 . (Review).
- Eva Halpick: Studies on the effect of amifostine (= WR (Walter Reed) -2721; S-2 (3-aminopropylamino) -ethyl phosphorothioate) on the clonogenic proliferation of freshly explanted human tumor cells in vitro. Dissertation, Ludwig Maximilians University Munich, 1998
- JL Mitchell, J. Rupert, A. Leyser, GG Judd: Mammalian cell polyamine homeostasis is altered by the radioprotector WR1065. In: The Biochemical journal , Volume 335, October 1998, pp. 329-334, PMID 9761731 . PMC 1219786 (free full text).
- M. Treskes, U. Holwerda, I. Klein, HM Pinedo, WJ van der Vijgh: The chemical reactivity of the modulating agent WR2721 (ethiofos) and its main metabolites with the antitumor agents cisplatin and carboplatin. In: Biochemical Pharmacology , Volume 42, Number 11, November 1991, pp. 2125-2130, PMID 1659819 .
Web links
- Ethyol at rxlist.com (English)
- Amifostine at drugs.com (English)
Individual evidence
- ↑ a b c d U. Holzgrabe, H. Szelényi: Amifostin monohydrate. In: F. von Bruchhausen, S. Ebel, E. Hackenthal, U. Holzgrabe (Eds.): Hagers Handbook of Pharmaceutical Practice. 5th edition, Verlag Springer, 1999, ISBN 3-540-62644-1 , pp. 61-62. limited preview in Google Book search
- ↑ a b Amifostine data sheet from Sigma-Aldrich , accessed on April 28, 2011 ( PDF ).
- ↑ a b c d e f g h i MSDS: Amifostine ( Memento from September 19, 2011 in the Internet Archive ) March 2004
- ↑ a b C. R. Culy, CM Spencer: Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. In: Drugs Volume 61, Number 5, 2001, pp. 641-684, PMID 11368288 . (Review).
- ↑ a b C. M. Spencer, KL Goa: Amifostine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential as a radioprotector and cytotoxic chemoprotector. In: Drugs Volume 50, Number 6, December 1995, pp. 1001-1031, PMID 8612469 . (Review).
- ↑ PM Calabro-Jones, JA Aguilera, JF Ward, GD Smoluk, RC Fahey: Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. In: Cancer research Volume 48, Number 13, July 1988, pp. 3634-3640, PMID 2837319 .
- ↑ GD Smoluk, RC Fahey, PM Calabro-Jones, JA Aguilera, JF Ward: Radioprotection of cells in culture by WR-2721 and derivatives: form of the drug responsible for protection. In: Cancer research Volume 48, Number 13, July 1988, pp. 3641-3647, PMID 2837320 .
- ↑ PM Calabro-Jones, RC Fahey, GD Smoluk, JF Ward: Alkaline phosphatase promotes radioprotection and accumulation of WR-1065 in V79-171 cells incubated in medium containing WR-2721. In: International journal of radiation biology and related studies in physics, chemistry, and medicine Volume 47, Number 1, January 1985, pp. 23-27, PMID 2982751 .
- ↑ a b c d e f g h i j k l D. W. Wilder: Amifostine in subcutaneous use in patients with head and neck tumors. Dissertation, University of Tübingen, 2006 urn : nbn: de: bsz: 21-opus-22357
- ↑ D. Citrin, AP Cotrim, F. Hyodo, BJ Baum, MC Krishna, JB Mitchell: Radioprotectors and mitigators of radiation-induced normal tissue injury. In: Oncologist Volume 15, Number 4, 2010, pp. 360-371, doi: 10.1634 / theoncologist.2009-S104 . PMID 20413641 . PMC 3076305 (free full text). (Review).
- ↑ a b c d e J. R. Kouvaris, VE Kouloulias, LJ Vlahos: Amifostine: the first selective-target and broad-spectrum radioprotector. In: The oncologist Volume 12, Number 6, June 2007, pp. 738-747, doi: 10.1634 / theoncologist.12-6-738 . PMID 17602063 . (Review).
- ↑ JD Cox, KK Ang: Radiation oncology - rationale, technique, results. Verlag Elsevier Health Sciences, 2003, ISBN 0-323-01258-2 , p. 42. Restricted preview in the Google book search for: JM Yuhas, JM Spellmann, F. Culo: The role of WR-2721 in radiotherapy and / or chemotherapy. In: LW Brady (Ed.): Radiation Sensitizers. Masson Publishing, 1980
- ↑ a b c M. I. Koukourakis: Amifostine in clinical oncology: current use and future applications. In: Anti-Cancer Drugs Volume 13, Number 3, March 2002, pp. 181-209, PMID 11984063 . (Review).
- ↑ MI Koukourakis: Amifostine: is there evidence of tumor protection? In: Seminars in Oncology Volume 30, Number 6 Suppl 18, December 2003, pp. 18-30, PMID 14727237 . (Review).
- ^ AD Sasse, LG Clark, EC Sasse, OA Clark: Amifostine reduces side effects and improves complete response rate during radiotherapy: results of a meta-analysis. In: International Journal of Radiation Oncology - Biology - Physics Volume 64, Number 3, March 2006, pp. 784-791, doi: 10.1016 / j.ijrobp.2005.06.023 . PMID 16198504 . (Review).
- ↑ a b J. M. Yuhas, JM Spellman, F. Culo: The role of WR-2721 in radiotherapy and / or chemotherapy. In: Cancer Clinical Trials Volume 3, Number 3, 1980, pp. 211-216, PMID 6254681 .
- ↑ WJ DeNeve, CK Everett, JE Suminski, FA Valeriote: Influence of WR2721 on DNA cross-linking by nitrogen mustard in normal mouse bone marrow and leukemia cells in vivo. In: Cancer Research Volume 48, Number 21, November 1988, pp. 6002-6005, PMID 2844397 .
- ↑ a b M. Treskes, LG Nijtmans, AM Fichtinger-Schepman, WJ van der Vijgh: Effects of the Modulating agent WR2721 and its main metabolites on the formation and stability of cisplatin-DNA Adducts in vitro in comparison to the effects of thiosulphate and diethyldithiocarbamate. In: Biochemical pharmacology , Volume 43, Number 5, March 1992, pp. 1013-1019, PMID 1313234 .
- ↑ a b c R. L. Capizzi: The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine. In: Seminars in Oncology , Volume 26, Number 2 Suppl 7, April 1999, pp. 3-21, PMID 10348255 . (Review).
- ↑ LM Shaw, AT Turrisi, DJ Glover, HS Bonner, AL Norfleet, C. Weiler, MM Kligerman: Human pharmacokinetics of WR-2721. In: International Journal of Radiation Oncology, Biology, Physics , Volume 12, Number 8, August 1986, pp. 1501-1504, PMID 3019968 .
- ↑ a b L. M. Shaw, D. Glover, A. Turrisi, DQ Brown, HS Bonner, AL Norfleet, C. Weiler, JH Glick, MM Kligerman: Pharmacokinetics of WR-2721. In: Pharmacology & Therapeutics , Volume 39, Numbers 1-3, 1988, pp. 195-201, PMID 2849123 . (Review).
- ↑ a b L. M. Shaw, HS Bonner, A. Turrisi, AL Norfleet, M. Kligermann: Measurement of S-2- (3-aminopropylamino) ethanethiol (WR-1065) in blood and tissues. In: Journal of Liquid Chromatography . Volume 9, Number 4, 1986, pp. 845-859. doi: 10.1080 / 01483918608076673
- ↑ JM Yuhas: Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2- (3-aminopropylamino) -ethylphosphorothioic acid. In: Cancer Research Volume 40, Number 5, May 1980, pp. 1519-1524, PMID 6245795 .
- ↑ JL Millar, TJ McElwain, RD Clutterbuck, EA Wist: The modification of melphalan toxicity in tumor bearing mice by s-2- (3-aminopropylamino) - ethylphosphorothioic acid (WR 2721). In: American Journal of Clinical Oncology , Volume 5, Number 3, June 1982, pp. 321-328, PMID 6282111 .
- ^ LC Washburn, JJ Rafter, RL Hayes: Prediction of the effective radioprotective dose of WR-2721 in humans through an interspecies tissue distribution study. In: Radiation Research Volume 66, Number 1, April 1976, pp. 100-105, PMID 1257403 .
- ↑ Apply the radio protector as a bolus. ( Memento of October 17, 2013 in the Internet Archive ) Praxis-Depesche 15, 1999, based on: W. Wagner, A. Radmard, G. Mansour, K. Schonekas, S. Zaknoen: Improved feasibility of amifostine application in radiotherapy by using a new administration schedule (meeting abstract). ( Memento of the original from May 1, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: 1999 ASCO Annual Meeting Meeting Abstract # 2348
- ↑ J. Langell, R. Jennings, J. Clark, JB Ward: Pharmacological agents for the prevention and treatment of toxic radiation exposure in spaceflight. In: Aviation, Space, and Environmental Medicine , Volume 79, Number 7, July 2008, pp. 651-660, PMID 18619123 . (Review).
- ↑ R. Cosar, V. Yurut-Caloglu, S. Eskiocak, A. Ozen, S. Altaner, K. Ibis, N. Turan, B. Denizli, C. Uzal, M. Saynak, S. Parlar, M. Caloglu , B. Uregen, Z. Kocak: Radiation-induced chronic oxidative renal damage can be reduced by amifostine. In: Medical Oncology [electronic publication before print] February 2011, doi: 10.1007 / s12032-011-9870-7 . PMID 21347716 .
- ↑ a b c Ethyol at RxList.com, August 18, 2008.
- ↑ JR Piper, CR Stringfellow, RD Elliott, TP Johnston: S-2- (omega-aminoalkylamino) ethyl dihydrogen phosphorothioates and related compounds as potential antiradiation agents. In: Journal of Medicinal Chemistry Volume 12, Number 2, March 1969, pp. 236-243, PMID 5783596 .
- ^ S. Wolff: Harvey Milton Patt, Radiology; Physiology: San Francisco. Retrieved April 29, 2011
- ^ HM Patt, EB Tyree, RL Straube, DE Smith: Cysteine Protection against X Irradiation. In: Science Volume 110, Number 2852, August 1949, pp. 213-214, doi: 10.1126 / science.110.2852.213 . PMID 17811258 .
- ^ A b W. McCulloch, B. Scheffler, P. Schein: WR-2721 (ETHYOL): Reduction in Toxicity of Anticancer Therapy Without Loss of Efficacy. SB Howell (Ed.): Platinum and other metal coordination compounds in cancer chemotherapy. Verlag Springer, 1991, ISBN 0-306-44027-X , pp. 509-516, limited preview in the Google book search
- ↑ P. Alexander, ZM Bacq, SF Cousens, M. Fox, A. Herve, J. Lazar: Mode of action of some substances which protect against the lethal effects of x-rays. In: Radiation research Volume 2, Number 4, June 1955, pp. 392-415, PMID 14385034 .
- ↑ ZM Bacq, P. Alexander: Importance for radio-protection of the reaction of cell to sulphydyrl and disulphide compounds. In: Nature , Volume 203, July 1964, pp. 162-164, PMID 14207236 .
- ↑ JM Yuhas, JB Storer: Differential chemoprotection of normal and malignant tissues. In: Journal of the National Cancer Institute Volume 42, Number 2, February 1969, pp. 331-335, PMID 5765464 .
- ^ RL Capizzi: Clinical status and optimal use of amifostine. In: Oncology Volume 13, Number 1, January 1999, pp. 47-59, PMID 10027198 . (Review).
- ↑ DJ Grdina, Y. Kataoka, JS Murley: Amifostine: mechanisms of action underlying cytoprotection and chemoprevention. In: Drug metabolism and drug interactions Volume 16, Number 4, 2000, pp. 237-279, PMID 11201306 .
- ↑ DE Davidson, MM Grenan, TR Sweeney: Biological characteristics of some improved radio protectors. In: LW Brady (editor): Radiation Sensitizers, Their use in the Clinical Management of Cancer. Volume 5 of Cancer Management Masson, 1980, ISBN 0-89352-112-4 , pp. 309-320.