Cysteamine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
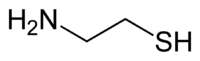
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Cysteamine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 7 NS | |||||||||||||||||||||
| Brief description |
colorless crystalline powder with an unpleasant odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 77.15 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
97-98.5 ° C |
|||||||||||||||||||||
| boiling point |
decomposes |
|||||||||||||||||||||
| solubility |
Easily soluble in water with an alkaline reaction, easily soluble in methanol and ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cysteamine is an organic chemical compound with the constitutional formula HSCH 2 CH 2 NH 2 . It is the simplest stable member of the aminothiol class . The hydrochloride with the formula C 2 H 7 NS · HCl with the melting point 70-71 ° C crystallizes from an aqueous solution in hydrochloric acid . Cysteamine is not to be confused with cystamine , which is derived from cystine and represents the disulfide of cysteamine.
Occurrence
In nature, the substance occurs as a degradation product through decarboxylation of the amino acid cysteine , which is why it also bears the common name decarboxycysteine . When coenzyme A is broken down, cysteamine is also formed.
Extraction and presentation
Cysteamine can be obtained by reacting aziridine with hydrogen sulfide .
structure
Cysteamine consists of a basic ethane structure which is substituted on both carbon atoms by a thiol or an amino group .
Chemical properties
It is used as the hydrochloride because the free thiol is easily oxidized to the corresponding disulfide . The oxidation of the cysteamine to the disulfide is facilitated by heating for a few hours at 80 ° C. with triethylamine in dimethylformamide under atmospheric oxygen. In addition, the reaction can be accelerated for a few minutes under ultrasound at room temperature.
biosynthesis
When coenzyme A is broken down , pantethein is initially formed , which is converted into pantothenic acid and cysteamine by the enzyme pantetheinase (also known as vanine) . Cysteamine, like cysteine, can be converted to hypotaurine by cysteamine dioxygenase and then oxidized to taurine by means of hypotaurine dehydrogenase . Then you can convert taurine to bile salts and, after the bile salts have passed through the intestinal-liver cycle , be excreted.
It is believed that less than 3% of the cysteamine formed is converted to methanethiol and acetamide with the help of a methyl transferase and subsequent conversion by cytochrome P450 . Subsequently, methanethiol is converted to dimethyl sulfide by another methyl transferase .
Biochemical and pharmaceutical use
Under the trade name Cystagon (approval in June 1997) or Procysbi ( retarded preparation: cysteamine hydrogen tartrate , approval in September 2013) it is used for the treatment of disorders of cystine excretion (nephropathic cystinosis and cystinuria ). In the human body, cysteamine is transported to the lysosomes , where it reacts with cystine, cleaving its disulfide bridge and thereby forming the disulfide with cysteine. This is much more soluble than cystine and is therefore more easily excreted through the kidneys. It is also used to treat radiation sickness due to its radical scavenger properties .
In the human organism, cysteamine is a component of coenzyme A, which contains other components such as β-alanine , pantoic acid (2,4-dihydroxy-3,3-dimethylbutyric acid), diphosphate and 3'-phosphorylated adenosine . Here, β-alanine and pantoic acid together form pantothenic acid . If one considers this together with cysteamine, one speaks of pantethein. Due to the thiol group (SH group) of the cysteamine part, the coenzyme A is able to enter into high-energy compounds. These compounds enter into with the carboxy groups (-COOH) of alkanoic and fatty acids to form thioester bonds .
In November 2008, the biopharmaceutical company Raptor Pharmaceutical (now Horizon Therapeutics ), in collaboration with the Center hospitalier universitaire d'Angers in France, announced that the treatment of Huntington's disease with the help of cysteamine hydrogen tartrate would be investigated more closely. After a three-year clinical study , the results were presented in December 2015 and it could not be proven that it led to improvements, but there was a slight delay in symptoms in isolated patients and no intolerant side effects. For the treatment of neuronal ceroid lipofuscinosis one is combination therapy with cysteamine bitartrate and N - acetylcysteine recommended. In the treatment of non-alcoholic steatohepatitis in children with cysteamine hydrogen tartrate, the alanine aminotransferase and aspartate aminotransferase concentrations are lowered and the risk of lobular inflammation of the liver is also reduced.
Individual evidence
- ↑ Safety data sheet . (PDF; 85,926 bytes) In: datasheets.scbt.com. Santa Cruz Biotechnology, Inc., p. 4 , accessed December 9, 2015 .
- ↑ a b c Entry on cysteamine in the Hazardous Substances Data Bank , accessed December 9, 2015.
- ↑ a b c Entry on cysteamine. In: Römpp Online . Georg Thieme Verlag, accessed on October 1, 2016.
- ↑ a b c d data sheet cysteamine (PDF) from Carl Roth , accessed on December 9, 2015.
- ^ Reid, E. Emmet: Organic Chemistry of Bivalent Sulfur . (PDF; 17,730 kB) In: Chemical Publishing Company . 1, No. 8, 1958, pp. 398-399.
- ↑ José Luis García Ruano, Alejandro Parra, José Alemán: Efficient synthesis of disulfides by air oxidation of thiols under sonication. In: Green Chemistry. 10, 2008, p. 706, doi : 10.1039 / B800705E .
- ↑ L. Gallego-Villar, Luciana Hannibal, J. Häberle, B. Thöny, T. Ben-Omran, GK Nasrallah, Al-N. Dewik, WD Kruger, HJ Blom: Cysteamine revisited: repair of arginine to cysteine mutations. In: Journal of Inherited Metabolic Disease. 40, 2017, p. 555, doi : 10.1007 / s10545-017-0060-4 .
- ^ WA Gahl, F. Tietze, JD Butler, JD Schulman: Cysteamine depletes cystinotic leucocyte granular fractions of cystine by the mechanism of disulphide interchange. In: Biochemical Journal. 228, 1985, p. 545, doi : 10.1042 / bj2280545 .
- ↑ BP Lukashin, AN Grebeniuk: Comparative study of the radiation-protective effectiveness of low doses of cysteamine, heparin, and naphtizine in experiments on mice . In: Radiats Biol Radioecol . 2001. PMID 11458646 .
- ↑ Raptor Pharmaceutical Completes DR Cysteamine Phase 2b Clinical Trial in Cystinosis. In: FierceBiotech. November 24, 2009, accessed July 7, 2019 .
- ^ Looking Past the Spin: Results from a Clinical Trial of Cysteamine. In: eurohuntington. January 11, 2016, accessed July 7, 2019 .
- ↑ Sondra W Levin, Eva H Baker, Wadih M Zein, Zhongjian Zhang, Zenaide MN Quezado, Ning Miao, Andrea Gropman, Kurt J Griffin, Simona Bianconi, Goutam Chandra, Omar I Khan, Rafael C Caruso, Aiyi Liu, Anil B Mukherjee : Oral cysteamine bitartrate and N-acetylcysteine for patients with infantile neuronal ceroid lipofuscinosis: a pilot study. In: The Lancet Neurology. 13, 2014, p. 777, doi : 10.1016 / S1474-4422 (14) 70142-5 .
- ↑ Jeffrey B. Schwimmer, Joel E. Lavine: In Children With Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but Does Not Reduce Disease Activity Scores. In: Gastroenterology. 151, 2016, p. 1141, doi : 10.1053 / j.gastro.2016.08.027 .



