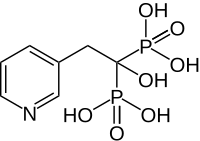
Risedronic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Risedronic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
fine, white to off-white, odorless, crystalline powder (risedronate sodium) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| solubility |
soluble in water; Essentially insoluble in common organic solvents (risedronate sodium) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Risedronic acid INN - also risedronate - ( trade name Actonel ) is a drug from the bisphosphonate group and is used as a sodium salt, especially for the treatment of osteoporosis .
Clinical information
Application areas (indications)
Risedronic acid is used as risedronate sodium in women to treat postmenopausal osteoporosis to reduce the risk of hip and vertebral fractures, and in men to treat osteoporosis when the risk of fractures is increased. In addition, there is a drug approval for increasing bone mass in high- risk patients who take systemic glucocorticoids in higher doses over a longer period of time .
Another approved use for risedronic acid is in the treatment of Paget's syndrome and pediatric osteogenesis imperfecta .
Contraindications (contraindications)
The use of risedronic acid is absolutely contraindicated in patients with known hypersensitivity to this active ingredient, in hypocalcaemia , during pregnancy and breastfeeding and in the presence of severe renal dysfunction with a creatinine clearance of less than 30 ml / min . As risedronic acid, like other bisphosphonates, has been associated with an increase in esophagitis , gastritis, and esophageal and gastroduodenal ulcers, precautions should be taken in patients with these diseases and in those who cannot remain in an upright position for at least 30 minutes after ingestion , to be hit.
Interactions
As with other drugs from the bisphosphonate group, simultaneous consumption of drugs and foods that contain polyvalent cations such as magnesium , calcium , iron and aluminum leads to a reduced absorption of the active ingredient. Concomitant use of nonsteroidal anti-inflammatory drugs such as acetylsalicylic acid , ibuprofen and diclofenac can increase the risk of gastrointestinal disorders .
Side effects
The most common gastrointestinal disorders such as constipation , indigestion , nausea , abdominal pain and diarrhea can be observed with a frequency of 1–10% during the use of risedronic acid . Occasionally (0.1–1%) gastritis , esophagitis , dysphagia , duodenitis, and esophageal ulcer occur , and rarely (0.01–0.1%) glossitis and esophageal stricture . In addition, headaches and pain in the skeletal and muscular area were often observed (1–10%) .
pharmacology
Pharmacodynamics (mechanism of action)
Risedronic acid, like other bisphosphonates, has two effects on the bones. Risedronic acid inhibits bone turnover by accumulating on the bone hydroxylapatite on the bone surface. In addition, bone loss is inhibited by inhibiting the osteoclasts .
Pharmacokinetics
After oral administration, only a relatively small part of the risedronate is absorbed into the systemic circulation. The systemic bioavailability is around 0.6%. There is no metabolism in the body. Risedronic acid is eliminated from the body in three phases. The terminal plasma half-life is over 400 hours. Therefore, a daily administration of the substance is not necessary if the individual dose is chosen accordingly.
literature
- P. Rackoff: Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis . In: Clinical Interventions in Aging . tape 4 , 2009, p. 207-214 , PMID 19503783 , PMC 2685242 (free full text).
Individual evidence
- ^ A b The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station, NJ, USA 2006, ISBN 0-911910-00-X .
- ↑ a b Registration dossier on 1-hydroxy-2- (3-pyridinyl) ethylidene-bisphosphonic acid ( GHS section ) at the European Chemicals Agency (ECHA), accessed on July 11, 2020.
- ^ T. Masud, M. McClung, P. Geusens: Reducing hip fracture risk with risedronate in elderly women with established osteoporosis . In: Clinical Interventions in Aging . tape 4 , 2009, p. 445-449 , PMID 19966913 , PMC 2785868 (free full text).
- ↑ a b c d Warner Chilcott Deutschland GmbH: Technical information Actonel ® once a week 35 mg film-coated tablets. As of March 2010. (No longer available online.) Formerly in the original ; Retrieved July 12, 2010 . ( Page no longer available , search in web archives )
- ↑ Warner Chilcott Deutschland GmbH: Technical information Actonel ® 5 mg film-coated tablets. As of March 2010. (No longer available online.) Formerly in the original ; Retrieved July 12, 2010 . ( Page no longer available , search in web archives )
- ↑ Warner Chilcott Deutschland GmbH: Technical information Actonel ® 30 mg film-coated tablets. As of March 2010. (No longer available online.) Formerly in the original ; Retrieved July 12, 2010 . ( Page no longer available , search in web archives )
- ^ AL Langston, SH Ralston: Management of Paget's disease of bone . In: Rheumatology . tape 43 , no. 8 , August 2004, p. 955-959 , PMID 15187244 .
- ↑ N. Bishop et al.: Risedronate in children with osteogenesis imperfecta: a randomized, double-blind, placebo-controlled trial. In: Lancet. 382, 2013, pp. 1424-1432, doi: 10.1016 / S0140-6736 (13) 61091-0 .
- ↑ Heinz Lüllmann, Klaus Mohr, Lutz Hein: Pharmacology and Toxicology. Stuttgart 2006, pp. 262-264.
- ↑ Christoffel Jos van Boxtel: Drug Benefits and Risks. IOS Press, 2008, ISBN 978-1-58603-880-9 , p. 399 ( limited preview in Google book search).
- ^ NB Watts, JP Brown, G. Cline: Risedronate on 2 consecutive days a month reduced vertebral fracture risk at 1 year compared with historical placebo . In: Journal of Clinical Densitometry . tape 13 , no. 1 , 2010, p. 56-62 , doi : 10.1016 / j.jocd.2009.09.005 , PMID 19942469 .
Web links
- Entries in the NIH study registry
- Recommendations of the umbrella organization for osteology (DVO) guideline: prophylaxis, diagnosis and therapy of osteoporosis in adults. ( Memento from September 11, 2010 in the Internet Archive )