Roseoflavin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
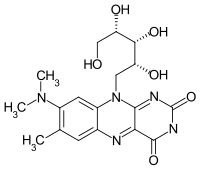
|
|||||||||||||
| General | |||||||||||||
| Surname | Roseoflavin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 18 H 23 N 5 O 6 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 405.41 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
276-278 ° C |
||||||||||||
| solubility |
very good in DMSO , soluble in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Roseoflavin ("Roseo-", English, prefix for rose-red ) is an organic compound that is structurally very similar to the growth substance vitamin B 2 ( riboflavin ), which can be isolated from the culture filtrate of the gram-positive soil bacterium Streptomyces davawensis . This pink or red pigment has an antibiotic effect on gram-positive bacteria such as Bacillus subtilis or Staphylococcus aureus . Roseoflavin is the only known, naturally occurring flavin analog with an antibiotic effect.
Occurrence
Roseoflavin is synthesized by the soil bacterium Streptomyces davawensis . Streptomyces davawensis was isolated from a Philippine soil sample as part of a "screening" program for antibiotics . Further research by a Japanese team of researchers revealed that Streptomyces davawensis produces a conspicuous pink organic compound and excretes it in the culture medium. The structure of the pink material was determined by means of NMR spectroscopy .
Extraction and presentation
Roseoflavin can be isolated from the culture supernatant of Streptomyces davawensis . Little is known about the biosynthesis of the compound. It is assumed, however, that roseoflavin is formed from GTP via riboflavin . In 2011 the first enzyme of roseoflavin biosynthesis was identified, a SAM- dependent methyltransferase that catalyzes the last two reactions from 8-amino-8-demethyl-D-riboflavin to roseoflavin. In this reaction, two methyl groups are successively transferred from two SAM molecules to the amino group on the C 8 atom .
Through various extraction and purification steps, dark red crystals can be obtained from the culture filtrate. The process of isolating roseoflavin from the culture supernatant is currently very complex. In addition, the yields of 0.5 mg / l culture supernatant are very low, so that commercially available roseoflavin is obtained by chemical synthesis .
properties
Roseoflavin forms dark red crystals and is odorless. Roseoflavin is photosensitive in both aqueous and organic solutions . When exposed to sunlight, it changes color from pink to yellow. Melting point: 276-278 ° C. UV / VIS - absorption spectrum : Wavelength (nm) ( molar extinction coefficient x 10 -3 ) 223 (14.0), 258 (40.0), 314 (6.77), 505 (32.8) roseoflavin is highly soluble in DMSO , soluble in water (approx. 250 µM), pyridine , methanol , acetone and n- butanol . Roseoflavin is not soluble in benzene , n -hexane , chloroform and carbon tetrachloride .
Biological importance
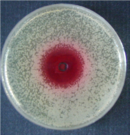
Roseoflavin has weak antibiotic effects against some gram-positive bacteria such as Staphylococcus aureus , Sarcina lutea , Bacillus cereus and Bacillus subtilis (see also antivitamin ). This antibiotic effect is due to the fact that roseoflavin acts as a riboflavin antagonist. Roseoflavin forms inactive cofactors for flavoenzymes ( FMN , FAD ) or acts on a so-called riboswitch . The exact mechanism is not yet known. Roseoflavin also inhibits the maturation of Plasmodium falciparum , the causative agent of malaria in humans.
Individual evidence
- ↑ a b c S. Otani et al .: Studies on roseoflavin: isolation, physical, chemical and biological properties. In: Flavins and flavoproteins. Volume 33, 1976, pp. 323-327.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ S. Otani, M. Takatsu, M. Nakano, S. Kasai, R. Miura: Letter: Roseoflavin, a new antimicrobial pigment from Streptomyces . In: Journal of Antibiotics . tape 27 , no. 1 , 1974, p. 86-87 , PMID 4843053 .
- ^ S. Otani, R. Miura, K. Matsui: Chemical Structure and Some Properties of Roseoflavin . In: Bulletin of the Chemical Society of Japan . tape 48 , no. 10 , 1975, p. 2877-2880 , doi : 10.1246 / bcsj.48.2877 .
- ↑ F. Jankowitsch, C. Kühm, R. Kellner, J. Kalinowski, S. Pelzer, P. Macheroux, M. Mack: A novel N, N-8-amino-8-demethyl-D-riboflavin dimethyltransferase (RosA) catalyzing the two terminal steps of roseoflavin biosynthesis in Streptomyces davawensis . In: Journal of Biological Chemistry . tape 286 , no. 44 , 2011, p. 38275-38285 , doi : 10.1074 / jbc.M111.292300 , PMID 21911488 , PMC 3207391 (free full text).
- ↑ S. Otani, S. Kasai, K. Matsui: Isolation, chemical synthesis, and properties of roseoflavin . In: Methods in Enzymology . tape 66 , 1980, pp. 235-241 , PMID 7374471 .
- ↑ S. Grill, S. Busenbender, M. Pfeiffer, U. Köhler, M. Mack: The bifunctional flavokinase / flavin adenine dinucleotide synthetase from Streptomyces davawensis produces inactive flavin cofactors and is not involved in resistance to the antibiotic roseoflavin . In: Journal of Bacteriology . tape 190 , no. 5 , 2008, p. 1546–1553 , doi : 10.1128 / JB.01586-07 , PMID 18156273 , PMC 2258686 (free full text).
- ↑ KF Blount, RR Breaker: Riboswitches as antibacterial drug targets . In: Nature Biotechnology . tape 24 , no. 12 , 2006, p. 1558-1564 , doi : 10.1038 / nbt1268 , PMID 17160062 .
- ^ Matthias Mack: Mikrobiologie in Mannheim ( Memento from September 22, 2011 in the Internet Archive ). In: horizons. No. 32, July 2008.
