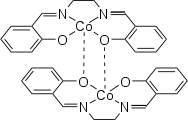
Salcomin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
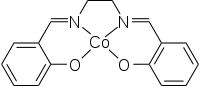
|
||||||||||||||||
| Crystal system |
monoclinic |
|||||||||||||||
| Space group |
C 2 / c (No. 15) |
|||||||||||||||
| Lattice parameters |
a = 26.380 (5) Å, b = 7.105 (5) Å, c = 14.470 (5) Å, β = 97.96 (2) ° |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Salcomin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 16 H 14 CoN 2 O 2 | |||||||||||||||
| Brief description |
red-brown, crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 325.21 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Salcomine (also: Co (salen) ) is a cobalt - complex . The compound was first synthesized by Paul Pfeiffer in 1933 .
etymology
The name Salcomin is a made-up word made up of the words salicylaldehyde , cobalt and ethylenediamine .
presentation
After synthesis of the salen - ligand by reacting ethylenediamine with salicylaldehyde in ethanol , the compound may as by the subsequent addition of a cobalt salt of cobalt (II) acetate are shown.
properties
The complex has a square-planar structure. The connection is paramagnetic , the effective magnetic moment is given in units of Bohr's magneton with 2.28 to 2.75 at room temperature.
Salcomin exists in an active and an inactive modification: The special property of the active form is that it is able to bind oxygen from the ambient air at room temperature (up to 4.9 % by weight of oxygen) and absorb this after heating 50–60 ° C to be returned. For this reason, the connection should be stored under protective gas .
Both modifications exist as dimers under standard conditions . In the active form there is a loose bond between the two cobalt atoms, while in the inactive form the oxygen atoms of the ligands bind to both cobalt atoms.
The inactive form crystallizes in the monoclinic space group C 2 / c (space group no.15 ) with the lattice constants a = 26.380 (5) Å, b = 7.105 (5) Å, c = 14.470 (5) Å, β = 97, 96 (2) ° and Z = 8.
use
On the Allied side, salcomin was used to produce oxygen during World War II .
The complex is also used as an initiator in free-radical polymerisation or as a chain transfer reagent . In organic chemistry, salcomin is used as a catalyst, for example in the oxidation of benzyl alcohol to benzaldehyde . The kinetic resolution can be carried out with chiral salcomin derivatives.
Individual evidence
- ↑ a b S. L. Holt, R. DeIasi, B. Post: Crystal structure of the oxygen-inactive form of bis (salicylaldehyde) ethylenediiminecobalt (II) . In: Inorganic Chemistry . tape 10 , no. 7 , July 1971, p. 1498-1500 , doi : 10.1021 / ic50101a036 .
- ^ A b Margot Becke-Goehring, Harald Hoffmann: Complex chemistry . Lectures on Inorganic Chemistry by Margot Becke-Goehring. Springer, 2013, ISBN 978-3-642-87215-0 , 2.10, pp. 93-94 ( limited preview in Google Book Search [accessed March 20, 2019]).
- ↑ a b Data sheet N, N'-Bis (salicylidene) ethylenediaminecobalt (II) from AlfaAesar, accessed on March 20, 2019 ( PDF )(JavaScript required) .
- ↑ a b c Data sheet N, N'-Bis (salicylidene) ethylenediaminocobalt (II) from Sigma-Aldrich , accessed on March 20, 2019 ( PDF ).
- ↑ a b c Entry on salcomin. In: Römpp Online . Georg Thieme Verlag, accessed on 2019-03-20.
- ↑ Patent EP0704428A1 : applied for on September 19, 1995 , published on April 3, 1996 , applicant: Evonik Degussa , inventor: Frank Dr. Hübner, Ulrich Gora, Klaus Dr. Huthmacher, Karlheinz Prof. Dr. Drrauz.
- ^ A b Trevor G. Appleton: Oxygen uptake by a cobalt (II) complex. An undergraduate experiment. In: Journal of Chemical Education . tape 54 , no. 7 July 1977, p. 443-444 , doi : 10.1021 / ed054p443 .
- ↑ J. Manasen: Structure of cobalt (II) complexes with quadridentate ship bases in solution and the solid state . In: Inorganic Chemistry . tape 9 , no. 4 , April 1970, p. 966-968 , doi : 10.1021 / ic50086a057 .
- ^ DN Kumar and BS Garg: Some new cobalt (II) complexes . Synthesis, characterization and thermal studies. In: Journal of Thermal Analysis and Calorimetry . tape 69 , no. 6 , August 2002, p. 607-616 , doi : 10.1023 / A: 1019976226610 .