Sulfur tetrafluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sulfur tetrafluoride | |||||||||||||||
| other names |
Sulfur (IV) fluoride |
|||||||||||||||
| Molecular formula | SF 4 | |||||||||||||||
| Brief description |
colorless gas with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 108.05 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.919 g cm −3 (liquid at −73 ° C) |
|||||||||||||||
| Melting point |
−121 ° C |
|||||||||||||||
| boiling point |
−40.4 ° C |
|||||||||||||||
| Vapor pressure |
10 bar (20 ° C) |
|||||||||||||||
| solubility |
Decomposes in water with violent reaction |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.1 ml m −3 or 0.4 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sulfur tetrafluoride is a chemical compound from the group of inorganic sulfur compounds and fluorides .
history
Sulfur tetrafluoride was discovered in 1929 by Joseph Fischer and Werner Jaenckner. The preparation was achieved from cobalt (III) fluoride and sulfur, with fluorspar being added to the reaction mixture to reduce the reactivity . The resulting sulfur tetrafluoride was condensed in liquid air .
Extraction and presentation
Sulfur tetrafluoride is made by direct fluorination of sulfur with fluorine over a narrow temperature range. The fluorination can also take place at −78 ° C in trichlorofluoromethane .
It can also be produced in the laboratory by reacting sulfur dichloride with sodium fluoride (or additionally with chlorine).
or according to a more recent reaction of bromine , sulfur and potassium fluoride :
properties
Sulfur tetrafluoride is a colorless, non-flammable gas with a pungent odor. It decomposes in water with violent reaction and when heated, producing hydrogen fluoride and sulfur dioxide . It has a critical temperature of 91 ° C, the triple point is at a temperature of −121 ° C and a pressure of 1.7 mbar. It acts as a weak Lewis acid and forms, for example, 1: 1 adducts with organic bases such as pyridine and triethylamine .
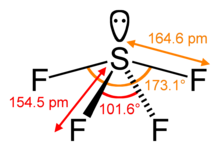
structure
In addition to its fluorine substituents, sulfur tetrafluoride has a non-bonding pair of electrons and thus altogether forms a distorted trigonal bipyramid based on the axial positions. The lone pair of electrons occupies one of the three equatorial positions and two fluorine substituents the other two. In the 19 F-NMR spectrum, however, only a single F signal is observed at room temperature, since all fluorine atoms change places too quickly.
use
Sulfur tetrafluoride is used as a fluorinating agent for inorganic oxides, sulfides or carbonyls or especially the keto group > C = O to> CF 2 .
Individual evidence
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 183-184.
- ↑ a b c d e f g h Entry on sulfur tetrafluoride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Data sheet sulfur tetrafluoride ( Memento of the original from October 23, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. at Multigas, accessed on October 23, 2016.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 7783-60-0 or sulfur tetrafluoride ), accessed on November 2, 2015.
- ↑ Joseph Fischer, Werner Jaenckner: A new fluorine-sulfur compound, the sulfur-4-fluoride (preliminary communication) . In: Journal for Applied Chemistry . tape 42 , no. 31 , August 3, 1929, pp. 810-811 , doi : 10.1002 / anie.19290423105 .
- ↑ D. Naumann 1, Dr. (Mrs.) DK Padma: The depiction of sulfur tetrafluoride from the elements at low temperature in an inert solvent. In: Journal of Inorganic and General Chemistry . 1973, 401, 1, pp. 53-56, doi: 10.1002 / zaac.19734010108 .
- ↑ Greenwood, Norman N .; Earnshaw, Alan: Chemistry of the Elements . tape 2 . Butterworth-Heinemann, 1997, ISBN 0-08-037941-9 .
- ↑ M. Pavone, V. Barone, I. Ciofini, C. Adamo: First-principle molecular dynamics of the Berry pseudorotation: insights on 19F NMR in SF4. In: The Journal of chemical physics. Volume 120, Number 19, May 2004, pp. 9167-9174, doi: 10.1063 / 1.1707012 , PMID 15267853 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 564.