Phase diagram

The phase diagram illustrates states and their associated phases in dependence on state variables (z. B. Pressure and temperature are). Alternative designations include state diagram , the state chart or equilibrium diagram .
It is a widely used tool in chemistry , physics and especially materials science . Phase diagrams are mostly used for solutions and alloys , but in principle also for any other type of substance or mixture of substances .
Phases are defined much more generally than the three states of aggregation (solid, liquid, gaseous) and therefore their synonyms only in special cases . There are various forms of appearance of phase diagrams, depending on how many substances, phases and variables are being considered. Phase transitions can also occur between different order states within a physical state .
Pure substance (single substance system)

A pT diagram for the three different aggregate states of a pure substance is very well suited to explain the scheme on which the phase diagrams are based. The diagrams contain some curves that delimit the areas of different phases, or here also states of aggregation. These curves, which are referred to as phase boundary lines , represent the mixed regions of these phases. Under the conditions given by them, several phases are in thermodynamic equilibrium . These areas are mathematically described by the Clapeyron equation and its modifications, especially the Clausius-Clapeyron equation for the phase transition condensed / gaseous. The lines are called the boiling point curve (between triple point and critical point , liquid / gaseous phase boundary), sublimation pressure curve (between zero point and triple point, solid / gaseous phase boundary), and melting pressure curve (solid / liquid phase boundary). Boiling point and sublimation pressure curves can also be combined to form the vapor pressure curve . According to Gibbs' phase rule, the critical point and the triple point have no degree of freedom , all lines have one degree of freedom (temperature or pressure) and there are two degrees of freedom (temperature and pressure) within the phase spaces.
The phase transitions of the water are of particular importance for the understanding of the dynamics within the atmosphere and thus of the weather in particular the humidity and the related cloud formation. It is therefore one of the most widely used phase diagrams, shown on the right in the lower phase diagram. It also has an important property that can only be observed in a minority of substances: the anomaly of water indicates that in the phase diagram the melting pressure curve leads from the triple point to lower temperatures with increasing pressure. It results from the physical properties of the water molecules and the resulting hydrogen bonds and is shown phenomenologically in the fact that ice has a lower density than water and can therefore float on it.
Multi-substance system
Especially in materials science or materials science , chemistry and process engineering , but also in geology , the multicomponent systems and, for reasons of illustration, especially the two-component systems, are of particular importance, with metals and their alloys or minerals being decisive . These are mixtures of substances made up of various components, which are described with regard to their mixing behavior in corresponding isobaric temperature - molar fraction phase diagrams ( isobaric change in state ) or temperature- mass fraction phase diagrams. To simplify matters, the following considerations are limited to two -component systems, whereby multicomponent systems can be described in isobaric and isothermal mole fraction triangular diagrams (three components), quadratic mole fraction square diagrams (four components) etc. or three-dimensional isobaric Tx phase diagrams (three components).
If two substances, usually of the same physical state, are mixed with one another, one or more mixed phases develop, which depend on the general miscibility of the substances, their respective concentration, pressure and temperature. Depending on can to get that only a mixed phase occurs in complete miscibility of the substances, or it forms two different mixed phases with limited solubility, which is a mixture gap referred. If the substances are not miscible at all, this miscibility gap extends over the entire Tp phase diagram.
Ideal binary mixture (two-component system)
In the case of an ideal mix, the miscibility gap always has a lens shape. Characteristics of ideal behavior are the absence of a change in volume or temperature during the mixing process. The mixing diagram (melting diagram) of forsterite ( ) and fayalite ( ) is an example of this, describing the composition of olivine .
On the left edge of such a diagram, the first component (A) is available as a pure substance, and consequently the second component (B) also on the right edge, which is why an approximately vertical limit ( segregation line) is drawn here to make it clear that the The abscissa is limited on both sides. The resulting curves, which meet at one point at the left and right edge, consequently describe the dependence of the phase transition temperatures on the composition of the mixed phase, with the edge points representing the respective transition temperature of the pure substances. In the case of pure substances, the transition temperatures are by definition independent of the direction of the phase change, which explains the outer points. However, if the substances occur in a mixture, the transition values differ, depending on whether the substance evaporates or condenses or solidifies or melts. Since all three states of aggregation only form a common equilibrium at the triple point, a distinction is made between the Tx diagrams according to the two states of aggregation occurring, whereby the solid-gaseous transition is not listed here due to its low relevance.
Melting diagram (solid-liquid)
The boundary line that delimits the liquid area is called the liquidus line and the one that delimits the solid area is called the solidus line . Above the liquidus line the substance is completely liquid (abbreviation: L), below the solidus line it is completely solid (abbreviation:) . The area between the liquidus and solidus lines is called the miscibility gap (abbreviation:) .
At high temperatures, the liquid phase normally has a lower Gibbs energy ( ) than the solid phase; this implies a minimum from a lower enthalpy H ( ) and / or higher entropy S . That is why it is "preferred" according to the laws of thermodynamics, and the solid phase melts completely in favor of the liquid. However, the lower the temperature, the better the energetic conditions for the solid phase, whereupon the liquid phase increasingly solidifies. In addition, the energetic "upper hand" depends on the mixture ratio of the substances, and consequently several corresponding mixed phases develop. Only when the temperature drops so far that the solid phase has the higher energy regardless of the mixing ratio, the isotherm is completely in the solid phase range of the diagram.
It should be noted that, without a temperature change, a dynamic equilibrium is established after a certain time (which in geology can be several million years) . The phase change processes balance each other out, but in principle still exist. This is particularly impressive with mixed crystals , which, when immersed in a miscibility gap, very slowly form different phases, which is known as segregation .
If the melt is cooled, part of the substance solidifies in a certain, temperature-dependent ratio. This ratio can be determined with the help of the Konode . In fact, there is no thermodynamically stable composition of the two substances in the miscibility gap that would correspond to the “inlet composition”. During cooling, the thermodynamically stable compositions of melt and solid phase move along the isotherm . The composition of the melt moves downwards along the liquidus line, while the composition of the solid phase also moves downwards parallel to the solidus line. Both always remain in the same isotherm, i.e. they are horizontally opposite in the Tx diagram. When the solid phase is melted, the same applies, only that the compositions move upwards in the diagram with increasing temperature. The temperatures traversed by the respective line are called the liquidus temperature or solidus temperature .
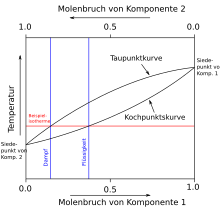
Boiling diagram (liquid-gaseous)
In contrast to the melting diagram, the upper line in the boiling diagram shows the condensation curve (also known as the dew line), while the lower line shows the boiling line. Consequently, the upper phase is gaseous and the lower phase is liquid. Everything else is analogous to the melting diagram. The temperature-molar fraction (Tx) or pressure-molar fraction diagrams (px diagrams) are used, for example, in the design and calculation of a distillation .
Real binary mix
Since real mixtures often behave differently than ideal mixtures, the phase diagrams can deviate from the idealized picture almost at will. Phase diagrams of eutectic compositions have a eutectic point . An example of this is the binary system of the minerals diopside - anorthite . Other terms belonging to the area are peritectic , dystectic and monotectic .
Unlimited miscibility gaps downwards (convex isobars) or less often upwards (concave isobars), the maxima and minima of which are referred to as the upper and lower critical separation points, are particularly common. An example of this is a mixture of phenol and water. One method for determining the composition of the individual phases of a two-component system is provided by the Konoden rule .
Ternary mixture (three-component system)
A system with three components is called a ternary system . Since these components are independent of each other, the composition of such a system is shown in an equilateral triangle, with each corner representing 100% of a particular component. Usage list is z. B. the representation of the composition of the earth's mantle in the system orthopyroxene , clinopyroxene and olivine .
As an example, an excerpt from the ternary system Au-Bi-Te can be seen. Such a ternary phase diagram can be imagined as three binary phase diagrams glued together at the end links, so here the diagrams of the systems Au-Bi, Bi-Te and Te-Au are connected in such a way that the corners of the end links Au-Au, Bi -Bi and Te-Te result. In order to display this 3D diagram correctly in 2D, it is viewed from above so that an equilateral triangle results. Usually the isolines and solidus are shown in different colors, here gray (isotherms) and red (solidus). The red arrows indicate the "topography" of the solidus.
See also
Web links
Explanations of the critical point, triple point, Gibbs' phase rule, state diagram of water and carbon dioxide, etc. a .:
- concise, but well explained, especially with regard to the degrees of freedom
- Mineral Atlas: phase diagram
- further phase diagrams of binary systems
Videos on boiling and melting diagrams:
- Video: Boiling line, dew line and azeotrope - How do you read boiling diagrams? . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15680 .
- Video: Conodes, Binodals and Invariant Points - How to Read Boiling Diagrams and Melting Diagrams? . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15225 .
Individual evidence
- ↑ according to Ciobanu et al. (2007)
- ↑ a b Jonas Börje Lundin: Investigation of bismut-bound gold mineralization near Vetlanda, southern Sweden . 2015, doi : 10.13140 / RG.2.1.4330.2483 ( researchgate.net [PDF; 9.6 MB ; accessed on September 14, 2017]).

![{\ mathrm {Mg_ {2} [SiO_ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9e12596bba5517169be036c5736c18553ee23734)
![{\ mathrm {Fe_ {2} [SiO_ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1dae45b44fee788f4847fae3a68c8f71a5ba27e5)