Strecker reduction
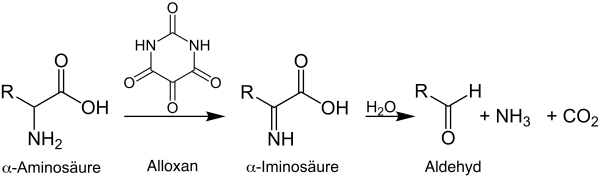
The Strecker degradation is a name reaction of organic chemistry , which was named after the German chemist Adolph Strecker . The reaction describes the irreversible, oxidative degradation of α- amino acids with α- dicarbonyl compounds to aldehydes or ketones , which are shortened by one carbon atom . In the original publication by Strecker, published in 1862, alloxan was used as an oxidizing agent and reacted with alanine , leucine and glycine .
As was observed later, the reaction can take place catalytically or non-catalytically, the non-catalyzed oxidation reactions being of greater importance. In addition, the latter can be carried out with a large number of organic or inorganic oxidizing agents . The organic oxidizing agents include, for. B. ketones , aldehydes or peroxycarboxylic acids , while ozone or hydrogen peroxide in the presence of silver oxide or iron (II) sulfate can act as inorganic oxidizing agents.
Reaction mechanism

Mechanistically, according to many authors (e.g. Zerong Wang ), the first step is the formation of an imine , which is formed by the nucleophilic reaction of the amino group from the α- amino acid with α- dicarbonyl compounds . This is followed by decarboxylation and hydrolysis of the imine, whereby the Strecker aldehyde or Strecker ketone and carbon dioxide shortened by one carbon atom are formed. Enaminols are formed as by-products, which can undergo subsequent reactions to form derivatives of pyridines , pyrazines or imidazoles . The reaction takes place more quickly at lower pH values because the carboxylate group protonates more quickly and carbon dioxide can therefore be eliminated more quickly .
Practical meaning
The Strecker degradation is also a substep in the Maillard reaction . The aldehydes resulting from the reaction form volatile aromatic components in foods. The breakdown of sulfur-containing amino acids (e.g. cystine , cysteine , methionine ) is particularly important for this , since sulfur-containing aldehydes such as methional are formed. The bond between the carbon - and sulfur atoms of these products is reductively easy to hydrogen sulfide , alkyl mercaptans , disulfides fissile and other connections. Highly diluted, these contribute to the aroma of many foods (e.g. onions , tomatoes , potatoes , mushrooms , meat , bread , coffee , etc.). The development of carbon dioxide during the Maillard reaction is attributed to Strecker degradation. Also for biochemical processes such as B. the development of the light taste of milk , the reaction is held responsible. In addition, the reaction can be used for the synthesis of aldehydes, ketones or α-aminoketones.
Individual evidence
- ^ Adolph Strecker: Note on a peculiar oxidation by alloxan . In: Annals of Chemistry . 123, No. 2, 1862, pp. 363-365. doi : 10.1002 / jlac.18621230312 .
- ↑ a b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents . 3rd volume. Wiley, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 2701-2706 .
- ↑ Alexander Schönberg , Radwan Moubacher: The Strecker Degradation of α-Amino Acids . In: Chem Rev.. . 50, No. 2, 1952, pp. 261-277. doi : 10.1021 / cr60156a002 .
- ^ Klaus Roth : Chemical delicacies . 1st edition. Wiley-VCH, Weinheim 2010, ISBN 978-3-527-32752-2 , pp. 108 .
- ↑ Thomas Hofmann, Petra Münch & Peter Schieberle: Quantitative model studies on the formation of aroma-active aldehydes and acids by Strecker-type reactions . In: Journal of agricultural and food chemistry . 48, No. 2, 2000, pp. 434-440. doi : 10.1021 / jf990954c .
- ↑ a b Michael Angrick, Dieter Rewicki: The Maillard reaction . In: Chemistry in Our Time . 14, No. 5, 1980, pp. 149-157. doi : 10.1002 / ciuz.19800140503 .
- ^ A b Ebermann, R. & Elmadfa, I .: Textbook Food Chemistry and Nutrition . 2nd Edition. Springer, Vienna 2011, ISBN 978-3-7091-0210-7 , pp. 108 .