Tannic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
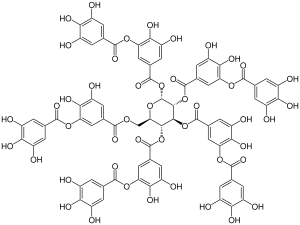
| Symbolic formula of a non-uniform mixture of structures | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tannic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 76 H 52 O 46 | ||||||||||||||||||
| Brief description |
light brown, odorless powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 1701.2 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.35 g cm −3 |
||||||||||||||||||
| boiling point |
218 ° C (decomposition) |
||||||||||||||||||
| solubility |
250 g l −1 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tannic acid , also called tannic acid or just tannin , is a chemically non-uniformly composed substance from the group of tannins . The polyphenolic structure is characteristic. Tannic acid does not contain any free carboxylic acid groups ( carboxy groups ), but is slightly acidic because of the large number of phenolic hydroxyl groups .
properties
Tannic acid is a mixture of esters of glucose with gallic acid and 3- galloylgallic acid . Formally, the substance around a glucose center contains 10 gallic acid units ( penta-digalloyl-glucose , see symbol formula on the right, corresponding to the empirical empirical formula C 76 H 52 O 46 ), but commercial tannic acid consists of molecules with 2–12 gallic acid residues. In pharmaceutical grades, there are on average 6 to 9 gallic acid residues for each glucose molecule.
It is a pale yellow amorphous powder or a shiny flaky or spongy solid that gradually darkens upon exposure to sunlight and air. Tannic acid is soluble in water, methanol , ethanol , acetone and ethyl acetate , but not in benzene , ether , chloroform , petroleum ether and carbon disulfide .
Tannic acid has an astringent effect . It has a low toxicity, but with increased oral intake it can lead to vomiting, diarrhea, abdominal pain and fatal circulatory disorders.
Industrial use
Tannic acid is used in many industrial areas:
- as a raw material for gallic acid and gallic acid pyrophosphate
- Mordant (tannic acid is often used in the preparation for electron microscopy to emphasize the fine structure of fibrillary elements (microtubules, microfilaments) or details of the plasma membrane (e.g. representation of the clathrin structure in the "coated pit").)
- for coloring
- Ore flotation agent
- Conversion coating
- Food additive
- deodorant
- Ink manufacturing
- Paper sizing
- Leather tanning
Use in medicine
Tannic acid is used locally, in the form of rinses (0.5 to 1%), ointments (5%) or brushes (10 to 20%) as an astringent - mainly on the mucous membranes.
The precipitation product of egg white or casein with tannin results in tannin protein (albumin tannate, tannin albuminate), an intestinal astringent that does not burden the stomach. It is indicated for the oral treatment of unspecific acute diarrhea , such as summer or traveler's diarrhea. The complex compound gelatin tannate can be used in the same indication. The compound silver protein acetyltannate is used to make eye and nose drops.
Oral administration for bleeding, chronic diarrhea, bloody urine, painful joints, persistent cough and cancer is mentioned on DrugBank . In the past, tannic acid was used as an antidote to various poisons.
- Finished preparations
Tannalbin (D), Tannacomp (D), Tasectan (CH)
- Formulation preparations according to the NRF
The collection of the New Formulation Form (NRF) contains a number of formulations in which tannic acid is processed for local treatment, sometimes in combination with other active ingredients such as betamethasone , triamcinolone , erythromycin or zinc oxide .
Individual evidence
- ↑ Entry on TANNIC ACID in the CosIng database of the EU Commission, accessed on December 29, 2019.
- ↑ a b c d Entry on tannin in the GESTIS substance database of the IFA , accessed on June 18, 2019 (JavaScript required)
- ↑ J. Falbe, M. Regitz (Ed.): Römpp Lexikon Chemie . 10th edition. 6 P-Z. Thieme, Stuttgart 1996. limited preview in Google book search.
- ↑ a b c Molecule of the Week Archive - Tannic acid. American Chemical Society , accessed June 5, 2019 .
- ↑ Tannic acid data sheet from Sigma-Aldrich , accessed on June 5, 2019 ( PDF ).
- ↑ K. Freudenberg: Tannin Cellulose · Lignin . Julius Springer Verlag, 1933, ISBN 978-3-642-47165-0 , p. 40 ff . ( limited preview in Google Book search).
- ^ A b T. Dingermann, Karl Hiller, G. Schneider, I. Zündorf: Schneider drug drugs. 5th edition. Elsevier, 2004. ISBN 3-8274-1481-4 . P. 321 f.
- ↑ a b c d Entry on Tannic acid at ChemicalBook , accessed June 5, 2019.
- ↑ David G. Robinson, Ulrich Ehlers, Rainer Herken, Bernd Herrmann, Frank Mayer, Friedrich-Wilhelm Schürmann: Preparation methodology in electron microscopy An introduction for biologists and medical professionals . Springer-Verlag, 2013, ISBN 978-3-662-09413-6 , pp. 29 ( limited preview in Google Book search).
- ↑ a b E. Teuscher: Biogenic medicines. 5th edition. Wissenschaftliche Verlagsgesellschaft, 1997. ISBN 3-8047-1482-X . P. 225.
- ↑ a b Entry on Tannic acid in the DrugBank of the University of Alberta , accessed June 5, 2019.
