Thiabendazole
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
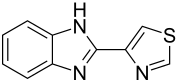
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Thiabendazole | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 10 H 7 N 3 S | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 201.24 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.4 g cm −3 |
|||||||||||||||||||||
| Melting point |
297-298 ° C |
|||||||||||||||||||||
| solubility |
heavy in water (<50 mg l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Thiabendazole (TBZ) is an active ingredient from the benzimidazole group , which is used as a fungicide and as an anthelmintic (worming agent). It was introduced by Merck, Sharp & Dohme in 1964 .
use
Agriculture
The substance is used as a systemic fungicide with protective and curative effects. As an Arbotect it was used against Dutch elm disease .
In Germany and Austria, thiabendazole is currently (as of 2016) only approved as an active ingredient in wound closure agents for trees.
In Switzerland, thiabendazole can also be used to treat seed potatoes . In addition, greenhouses in Switzerland to combat gray mold rot ( Botrytis cinerea ) can be smoked with a preparation containing thiabendazole.
Pesticides
Thiabendazole was removed from the list of additives in 1998 and has been listed as a fungicide ever since. As a result, the E number 233 became the INS number 233. This does not change anything in terms of the insert: it is added to the waxes with which the peel of citrus fruits and bananas are treated. Here it is supposed to prevent the formation of mold . The note “preserved with thiabendazole” is required for citrus fruits, but not for bananas, although the intake is mainly done by peeling with the hands; however, this information is often provided voluntarily. Furthermore, thiabendazole may be contained in fruit juices in extremely low concentrations.
Imazalil , orthophenylphenol or biphenyl are also used to prevent citrus fruits from going moldy .
The Tobacco Ordinance allows tobacco foil to be preserved with thiabendazole.
Veterinary medicine
In veterinary medicine , thiabendazole is a common wormer, which is mainly used against nematodes . It is given orally. The mechanism of action has not yet been finally clarified.
Analytics
The reliable qualitative and quantitative determination of thiabendazole succeeds after appropriate sample preparation by coupling the HPLC with the mass spectrometry . Gas chromatography with mass spectrometry coupling is also suitable for the determination of thiabendazole.
toxicology
The mean LD 50 (lethal dose) in cattle is 700 mg · kg −1 body weight, and in sheep 1200 mg · kg −1 body weight after oral administration. At similarly high doses, severe liver and kidney damage and death have been observed in dogs. Symptoms of thiabendazole poisoning may include palpitations, incoordination, ataxia , excessive salivation, and diarrhea.
According to the Federal Institute for Consumer Health Protection and Veterinary Medicine , the acute toxicity of thiabendazole is low overall . There is no evidence of a carcinogenic, mutagenic, or toxic effect on humans.
Individual evidence
- ↑ Entry on THIABENDAZOLE in the CosIng database of the EU Commission, accessed on April 29, 2020.
- ↑ a b c d e Entry on thiabendazole in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Entry on thiabendazole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data thiabendazole at Sigma-Aldrich , accessed on 24 April 2011 ( PDF ).
- ↑ Entry on thiabendazole. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 148-79-8 or thiabendazole ), accessed on November 2, 2015.
- ↑ Entry on thiabendazole in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on thiabendazole in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved February 19, 2016.
- ↑ EU Directive 98/72 (PDF) .
- ↑ Yoshioka N, Hayashi S, Inada T: Rapid Determination of Seven Fungicides in Citrus Fruits. , Shokuhin Eiseigaku Zasshi. 2015; 56 (5): 228-32, PMID 26537653
- ↑ Nagashima H, Hirao A, Tokuda Y, Uruta K: Simultaneous Determination of Seven Kinds of Fungicides in Citrus Fruits by Gas Chromatograghy / Mass Spectrometry. , Shokuhin Eiseigaku Zasshi. 2016; 57 (4): 101-6, PMID 27558228
- ↑ entry on thiabendazole at Vetpharm, accessed on 13 May, 2010.
- ↑ Statement of the Federal Institute for Consumer Health Protection and Veterinary Medicine on thiabendazole on fruit, fruits and in fruit juices ( Memento of June 12, 2007 in the Internet Archive ) (PDF; 14 kB).