1,5-cyclooctadiene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,5-cyclooctadiene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 12 | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 108.18 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.88 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−70 ° C |
|||||||||||||||
| boiling point |
150 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
very bad in water (<500 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4905 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,5-Cyclooctadiene is an organic compound with the empirical formula C 8 H 12 . It consists of an eight-membered ring which is doubly unsaturated and, as a cyclic diene, belongs to the cycloalkenes . The double bonds are not conjugated . 1,5-Cyclooctadiene is abbreviated as COD , especially in complexes .
presentation
1,5-cyclooctadiene, by dimerization of 1,3-butadiene in the nickel catalyst- bis [cyclooctadiene (1.5)] - nickel (0) are prepared. The worldwide annual production is around 10,000 tons.
Connections and reactions
It can be used as a labile bidentate chelating ligand in transition metal complexes. Here, 1,5-cyclooctadiene binds via both double bonds to the metal center. COD can easily be substituted by other ligands , which is why COD complexes can be used as starting materials for complex synthesis.
- Manufacture of nickel tetracarbonyl from Ni (COD) 2 and carbon monoxide .
Ni (COD) 2 can be prepared by reduction of nickel acetylacetonate with trimethylaluminum be prepared and COD.
A number of low-valent transition metal complexes with COD are known, of which the Crabtree catalyst is of greater importance .
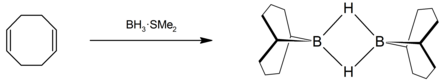
9-BBN , which is used for hydroboration , is produced by adding COD to a monoborane adduct.
Web links
Individual evidence
- ↑ a b c d e f g h i Entry on Cycloocta-1,5-diene in the GESTIS substance database of the IFA , accessed on September 29, 2019(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Thomas Schiffer, Georg Oenbrink: Cyclododecatriene, Cyclooctadiene, and 4-Vinylcyclohexene , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH, Weinheim 2005.
- ↑ R. Schunn, S. Ittel, Bis (1,5-Cyclooctadiene) Nickel (0) , in: Inorg. Synth. , 1990 , 28 , p. 94; doi : 10.1002 / 9780470132593.ch25 .
- ↑ John A. Soderquist, Alvin Negron: 9-Borabicyclo [3.3.1] nonane, dimer In: Organic Syntheses . 70, 1992, p. 169, doi : 10.15227 / orgsyn.070.0169 ; Coll. Vol. 9, 1998, p. 95 ( PDF ).




![{\ displaystyle \ mathrm {[Ni (C_ {5} H_ {7} O_ {2}) _ {2}] _ {3} \ + \ 6 \ COD \ + \ 6 \ Al (C_ {2} H_ { 5}) _ {3} \ rightarrow}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6ff7438b2cf82a58a3bb7f5f1407dbaca3fa7b6a)