10-undecen-1-ol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 10-undecen-1-ol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 22 O | |||||||||||||||
| Brief description |
clear colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 170.29 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−3 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
practically insoluble in water, soluble in alcohols such as ethanol , in diethyl ether , in chlorinated hydrocarbons such as dichloromethane and tetrahydrofuran , and in pyridine |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
10-Undecen-1-ol is a naturally occurring, linear alkenol with a terminal ethenyl group , which is obtained by reducing undecylenic acid or 10-undecenoic acid methyl ester - which in turn is easily accessible by cleaving castor oil .
Natural occurrence
10-Undecen-1-ol is found in the oil of Litsea odorifera Valeton , a laurel plant native to Indonesia .
Manufacturing
Reduction of 10-undecenoic acid with lithium aluminum hydride in diethyl ether gives 10-undecenol in practically quantitative yield.
Ethyl undecenoate is converted into 10-undecenol by Bouveault-Blanc reduction with metallic sodium and ethanol in yields of up to 82% of theory. receive.
The methyl ester of 10-undecenoic acid can also be gently reduced to 10-undecenol in 78% yield with titanium tetraisopropoxide (TIPT) and polymethylhydrosiloxane (PMHS).
Another related alternative is the reaction of the methyl ester with titanium tetraisopropoxide (TIPT) and triethoxysilane for 16 hours at 50 ° C. and subsequent hydrolysis with sodium hydroxide. The yield is 87% of theory.
properties
In its pure state, 10-undecen-1-ol is a clear, colorless liquid that solidifies to a waxy mass at temperatures below −3 ° C. Its smell is described as soapy, waxy, floral rose aroma.
Applications
The reaction of 10-undecen-1-ol with tosylated β-cyclodextrin produces a β-cyclodextrin undecenyl ether that can be covalently fixed as a monolayer on an etched glass surface , with the C 11 hydrocarbon chain acting as a hydrophobic spacer .
Cholesterol from milk forms inclusion compounds with the immobilized β-cyclodextrin through storage in the hydrophobic cavity. 73.6% of the cholesterol is extracted from the milk at 25 ° C. within 4 hours; the highest value achieved so far for such a test setup.
The radical addition of bifunctional thiols to 10-undecen-1-ol results in telechelic monodisperse mercapto alcohols which can be used as precursors for polyesters, polyurethanes and polyamides.
A quantitative yield of the telechelic mercapto alcohol can be achieved with the peroxyester t-butyl peroxypivalate as a radical starter and by adding dropwise the 10-undecenol to a large excess of 2-mercaptoethyl ether (2,2'-oxydiethanethiol).
10-Undecen-1-ol reacts with carbon monoxide in the presence of alkoxycarbonylation catalysts, such as. B. dicobalt octacarbonyl Co 2 (CO) 8 and a nitrogen base, such as. B. pyridine at pressures> 150 bar and temperatures in the range from 100 to 200 ° C to linear and branched polyesters with relatively high molecular weights (M n > 10,000 g / mol) in good yields.
The polyesters obtained are biodegradable and suitable for the production of films, fibers and moldings.
In a thiol-ene reaction ( click chemistry ) of 10-undecen-1-ol with mercapto alcohols, such as mercaptoethanol or 3-mercaptopropanol, α, ω-diols can be produced which are suitable for the synthesis of thermoplastic polyesters or polyurethanes.
In the case of light, possibly with the aid of photoinitiators , such as. B. Dimethoxyphenylacetophenon (DMPA), triggered radical addition of the thiol group to the terminal double bond, thioethers are formed almost quantitatively .
10-Undecenyl acrylate is obtained by reacting 10-Undecen-1-ol with acryloyl chloride and triethylamine in a yield of 78% of theory. receive.
In the presence of Grubbs catalysts , undecenyl acrylate (C 14 molecule) reacts as a quasi-α, ω-diene in an acyclic diene metathesis ADMET reaction with head-to-tail linkage and elimination of equimolar amounts of ethene to give the corresponding poly-diene with C 12 - Repetition unit.
The homopolymerization of 10-undecen-1-ol as a long-chain and sterically demanding olefin with a polar end group presents considerable difficulties. It is possible with metallocene catalysts, in particular with rac- ethylene bis [indenyl] zirconium dichloride, and the cocatalyst (activator) methylaluminoxane , the monomer protected by reaction with triisobutylaluminum to 10-undecenyloxy-diisobutylaluminum under optimal conditions with modest yields of 50 to 60 % and low molar masses M 4 <10 4 g / mol to convert to predominantly isotactic macromonomers.
The copolymerization of protected 10-undecen-1-ol with 10-undecene produces random copolymers with low molecular weights (M n <10 4 g / mol), while the copolymerization of allyl-terminated poly (10-undecen-1-ol) with propene does not clearly identifiable products (either copolymers with little incorporated polyundecenol or polymer blend of polypropylene and polyundecenol).
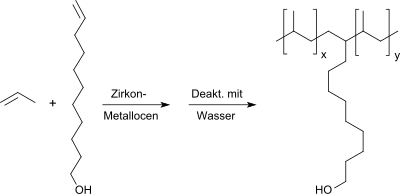
10-Undecen-1-ol can be incorporated into polypropylene to produce more hydrophilic polymer surfaces and thus improved adhesive properties through copolymerization with propene using zirconium metallocene catalysts,
however, because of the low tendency of the long-chain olefin alcohol to polymerize, only 0.1–0.9 mol% or 0.5.– 3.6% by weight.
The incorporation of 10-undecen-1-ol into copolymers with ethene with higher molar masses and contents of hydroxyl groups succeeds with phenoxy-based zirconium complexes.
Newer complexes of vanadium (III) chloride enable the production of copolymers of ethene and protected 10-undecen-1-ol reacted with triethylaluminum under mild conditions, which contain up to 15.8 mol% of the long-chain alcohol.
The new vanadium complexes are described as more effective copolymerization catalysts than the zirconium metallocenes previously used.
Because of its easy accessibility from the vegetable oil castor oil, which is available in abundance and inexpensively and which, as a renewable raw material, does not compete with food production, 10-undecen-1-ol has a certain potential as a future biogenic synthesis building block.
Individual evidence
- ↑ a b c d e f data sheet 10-Undecen-1-ol 99% at AlfaAesar, accessed on November 22, 2014 ( PDF )(JavaScript required) .
- ↑ a b c d e f g Data sheet 10-Undecen-1-ol 98% from Sigma-Aldrich , accessed on November 22, 2014 ( PDF ).
- ↑ a b W.M. Haynes: Handbook of Chemistry and Physics, 95th ed., 2014-2015 . CRC Press, Boca Raton, 2014, ISBN 978-1-4822-0867-2 , pp. 3-544 .
- ^ A b L. Montero de Espinosa, MAR Meier: Synthesis of star and block copolymers using ADMET: head-to-tail selectivity during step-growth polymerization . In: Chem. Commun. tape 47 , 2012, p. 1908-1910 , doi : 10.1039 / C0CC04161K .
- ↑ a b M.N. Tahir, C. Kwon, D. Jeong, E. Cho, SR Paik, S. Jung: Cholesterol reduction from milk using β-cyclodextrin immobilized on glass . In: J. Dairy Sci. tape 96 , 2013, p. 4191-4196 , doi : 10.3168 / jds.2012-6355 .
- ↑ P. van Romburgh: The essential oil Litsea odorifera Val. (Trawas oil) Prel. Comm. In: KNAW Proceedings . 14 I, 1911 ( PDF ).
- ↑ Patent US3941884 : Piperonyl ethers having juvenile hormone mimetic activity. Applied January 10, 1974 , published March 2, 1976 , applicant: Chevron Research Co., inventor: LH Edwards.
- ↑ DGM Diaper: Preparation of undecenol and undecenyl bromide . In: Canadian Journal of Chemistry . 39 (8), 1961, pp. 1723-1727, doi : 10.1139 / v61-220 .
- ↑ MT Reding, SL Buchwald: An inexpensive air-stable titanium-based system for the conversion of esters to primary alcohols . In: J. Org. Chem. Band 60 , no. 24 , 1995, pp. 7884-7890 , doi : 10.1021 / jo00129a031 .
- ↑ GL Larson, JL Fry: Ionic and organometallic-catalyzed organosilane reductions . Wiley, 2010, ISBN 978-0-470-54787-8 , pp. 132 .
- ↑ Product Information ( Memento from December 5, 2014 in the Internet Archive )
- ↑ B. Ameduri, K. Berrada, B. Boutevin, RD Bowden: Synthesis of a telechelic monodispersed mercapto-alcohol . In: Polymer Bull. Band 31 , no. 1 , 1993, p. 1-7 , doi : 10.1007 / BF00298756 .
- ↑ Patent EP2258743A2 : Manufacture of polyesters from renewable raw materials. Registered on June 2, 2010 , published on December 8, 2010 , applicant: BASF SE, inventor: TH Steinke, H.-H. Görtz, S. Mecking, D. Quinzler.
- ↑ CE Hoyle, CN Bowman: Thiol-En-Klickchemie . In: Angew. Chem. Band 122 , no. 9 , 2010, p. 1584-1617 , doi : 10.1002 / anie.200903924 .
- ↑ C. Pang, J. Zhang, G. Wu, Y. Wang, H. Gao, J. Ma: Renewable polyesters derived from 10-undecenoic acid and vanillic acid with versatile properties . In: Polym. Chem. Band 5 , 2014, p. 2843-2853 , doi : 10.1039 / C3PY01546G .
- ↑ M. Johannsen: Metallocene-catalyzed synthesis of polar olefin-based macromonomers . Dissertation at the Technical University of Dresden. 2011 ( online [PDF]).
- ↑ S. Paavola, R. Uotila, B. Löfgren, JV Seppälä: Enhanced adhesive properties of polypropylene through copolymerization with 10-undecen-1-ol . In: React Funct Polym . tape 61 , no. 1 , 2004, p. 53-62 , doi : 10.1016 / j.reactfunctpolym.2004.03.009 .
- ↑ X. Zhang, S. Chen, H. Li, Z. Zhang, Y. Lu, C. Wu, Y. Hu: Highly active copolymerization of ethylene with 10-undecen-1-ol using phenoxy-based zirconium / methylaluminoxane catalysts . In: J. Polym. Sci. A polym. Chem. Band 43 , no. 23 , 2005, pp. 5944-5952 , doi : 10.1006 / pola.21105 .