10-undecin-1-ol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| General | ||||||||||
| Surname | 10-undecin-1-ol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 11 H 20 O | |||||||||
| Brief description |
colorless to light yellow liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 168.28 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density | ||||||||||
| boiling point | ||||||||||
| solubility |
practically insoluble in water, soluble in alcohols such as methanol and ethanol , in chlorinated hydrocarbons such as dichloromethane and chloroform , and in tetrahydrofuran |
|||||||||
| Refractive index |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
10-Undecin-1-ol is a naturally occurring, linear alkynol with a terminal ethynyl group . It is easily accessible synthetically from 10-undecen-1-ol or 10-undecinic acid or methylundecinoate.
Occurrence
10-Undecin-1-ol occurs in considerable amounts (10.74%) in the pseudobulbs of Bulbophyllum kaitense Reichb.f. , a species of epiphytes native to India , which are used in traditional medicine ( Ayurveda ) and local natural medicine treatments as anti-inflammatory and antimicrobial medicinal drugs .
Manufacturing
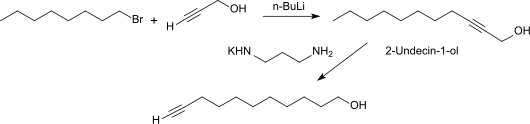
Reduction of 10-undecynic acid with lithium aluminum hydride in diethyl ether gives 10-undecynol in a yield of 89% of theory.
10-Undecynol can be obtained in 93% yield by isomerizing 2-Undecyn-1-ol (from the reaction of propargyl alcohol with n-Octyllithium).
The displacement of the internal triple bond to the chain end succeeds smoothly with the potassium amide of 1,3-diaminopropane .
Analogously to the preparation of 10-undecynoic acid from undecylenic acid , 10-undecyn-1-ol can be obtained from 10-undecen-1-ol by bromination of the double bond and subsequent double dehydrobromination with sodium amide in liquid ammonia in 60% yield.
Another synthetic route starts from the methyl ester of 10-undecynoic acid, which is reduced to 10-undecynol in 63% yield with tetraisopropyl orthotitanate and polymethylhydrosiloxane (PMHS).
properties
In its pure state, 10-Undecin-1-ol is a clear, colorless liquid that solidifies to a waxy mass at temperatures below 5 ° C. Like undecylenic acid, 10-undecinol also has a fungicidal effect, although this is limited by its even lower solubility in water. Inclusion compounds with methylated β-cyclodextrin increase the water solubility and thus the bioavailability of such solubilized 10-undecinol, which lead to a considerably increased effectiveness against phytopathogenic fungi such as Rosellina necatrix .
Applications
10-Undecinol is an important component in a synthesis route to the insect pheromone bombykol , which acts as a sexual attractant in the silk moth. A convergent stereospecific synthesis is based on the starting materials 10-undecynol and 1-pentyne .
In analogy to the preparation of 10,12-docosadiyne-1,22-diacid from 10-undecynoic acid by oxidative Glaser coupling , 10-undecin-1-ol can be obtained in the variant of the Eglinton reaction with copper (II) acetate in pyridine and catalytic amounts of copper (I) chloride in 68% yield to the corresponding α, ω-diol 10,12-docosadiyne-1,22-diol.
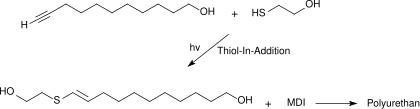
The simple thiol-in addition of mercaptoethanol to the ethynyl group of 10-undecynol leads to a vinyl thioether derivative which, as a diol component, reacts with MDI ( methylenediphenyl isocyanate ) to form linear polyurethanes.
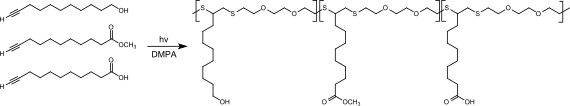
The terminal ethynyl group makes 10-undecynol a suitable molecule for click chemistry , e.g. B. for thiol-in coupling reactions. 10-undecinol (together with 10-undecinic acid and 10-undecinic acid methyl ester) reacts in a photoinitiated thiol-in reaction with 3,6-dioxa-1,8-dithiol in the presence of the photoinitiator DMPA (2,2-dimethoxy-2- phenylacetophenone) to comb-like oligothioethers, which can be used as functional polyols for polyurethane syntheses.
Another click reaction, the copper (I) iodide- catalyzed azide-alkyne cycloaddition (CuAAC, copper (I) -catalyzed azide-alkyne cycloaddition ) leads to the conversion of TBDMS ( tert- butyldimethylsilyl) -protected glutaric acid half-ester of 10-undecinol with the bis- azide 1,5-diazido-3-oxapentane [bis (2-azidoethyl) ether] in 86% yield to substituted 1,2,3-triazoles .
The resulting polymerizable bolaamphiphil can serve as a model compound for membrane-spanning lipids, which give the membranes of the archaea's biological domain extreme stability against high thermal (up to 121 ° C), osmotic (approx. 30% NaCl solution) and hydrolytic (pH 0) stress . The length of the hydrophobic chain between the two hydrophilic head groups of the bistriazole bolaamphiphile should be sufficient to span the approximately 35 angstrom thick lipid bilayer of biomembranes.
Individual evidence
- ↑ a b c d data sheet 10-Undecyn-1-ol from AlfaAesar, accessed on October 29, 2014 ( PDF )(JavaScript required) .
- ↑ a b c d Data sheet 10-Undecyn-1-ol, ≥95.0% (GC) from Sigma-Aldrich , accessed on December 26, 2014 ( PDF ).
- ↑ a b c L.D. Bergel'son, YG Molotkovskii, MM Shemyakin: Unsaturated acids and macrocyclic lactones: I. Synthesis of diacetylenic and dienic macrocyclic lactones . In: Zh. Obshch. Khim. tape 32 , 1962, pp. 58-64 (CA: 57, 14930 (1962)).
- ↑ S. Inayama, T. Tatewaki, S. Okada: Solid-state polymerization of conjugated hexayne derivatives with different end groups . In: Polymer J. Band 42 , 2010, p. 201–207 , doi : 10.1038 / pj.2009.326 .
- ↑ H. Woo, Y. You, T. Kim, G.-J. Jhon, W. Nam: Fluorescence ratiometric zinc sensors based on controlled energy transfer . In: J. Mater. Chem. Band 22 , 2012, p. 17100 , doi : 10.1039 / c2jm33366j .
- ↑ GW Kabalka, M. Varma, RS Varma, PC Srivastava, FF Knapp Jr .: tosylate ion of alcohols . In: J. Org. Chem. Band 51 , no. 12 , 1986, pp. 2386-2388 , doi : 10.1021 / jo00362a044 .
- ↑ A. Kaliarasan, SA John: GC-MS Analysis of Bulbophyllum Kaitense Rechib, pseudobulbs eastern ghats of India. . In: Int. J. Chem. Appl. tape 3 , no. 3 , 2011, p. 215–220 ( PDF ( Memento of February 16, 2015 in the Internet Archive )).
- ↑ H. Winarno: Rapid Isomerization of alkynol by potassium aminopropylamide reagent . In: Indo. J. Chem. Volume 7 , no. 3 , 2007, p. 320-323 ( online ).
- ^ L. Brandsma: Preparative Acetylenic Chemistry . In: Studies in Organic Chemistry 34 . Elsevier, 1988, ISBN 0-444-42960-3 , pp. 245-246 .
- ↑ a b H. Bader, H. Ringsdorf: Liposomes from α, ω- dipolar amphiphiles with a polymerizable diyne moiety in the hydrophobic chain . In: J. Polym. Sci., Polym. Chem. Ed. tape 20 , no. 6 , 1982, pp. 1623–1628 , doi : 10.1002 / pol.1982.170200622 .
- ↑ MT Reding, SL Buchwald: An inexpensive air-stable titanium-based system for the conversion of esters to primary alcohols . In: J. Org. Chem. Band 60 , no. 24 , 1995, pp. 7884-7890 , doi : 10.1021 / jo00129a031 .
- ↑ TL Neoh, T. Tanimoto, p Ikefuji, H. Yoshi, T. Furuta: Improvement of antifungal activity of 10-undecyne-1-ol by inclusion complexation with cyclodextrin derivatives . In: J. Agric. Food Chem. Band 56 , no. 10 , 2008, p. 3699-3705 , doi : 10.1021 / jf.0731898 .
- ↑ T. Sakurai, T. Nakagawa, H. Mitsuno, H. Mori, Y. Endo, S. Tanoue, Y. Yasukochi, K. Touhara, T. Nishioka: Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori . In: Proc. Natl. Acad. Sci. tape 101 , no. 47 , 2004, p. 16653-16658 , doi : 10.1073 / pnas.0407596101 .
- ↑ N. Miyaura, H. Suginome, A. Suzuki: New stereospecific syntheses of pheromone bombykol and its three geometrical isomers . In: Tetrahedron . tape 39 , no. 20 , 1983, p. 3271-3277 , doi : 10.1016 / S0040-4020 (01) 91575-3 .
- ^ RJ González-Paz, G. Lligadas, JC Ronda, M. Galià, V. Cádiz: Thiol-yne reaction of alkyne-derivatized fatty acids: Thiol-reactive linear polyurethane . In: J. Renew. Mater. tape 1 , 2013, p. 187 , doi : 10.7569 / JRM.2013.634114 .
- ^ R. Hoogenboom: Thiol-yne chemistry: A powerful tool for creating highly functional materials . In: Angew. Chem. Int. Ed. tape 49 , no. 20 , 2010, p. 3415–3417 , doi : 10.1002 / anie.201000401 .
- ↑ C. Lluch, G. Lligadas, JC Ronda, M. Galià, V. Cádiz: Thiol-yne approach to biobased polyols: Polyurethane synthesis and surface modification . In: abiosus eV, 6th Workshop on fats and oils as renewable feedstock for the chemical industry . 2013, p. 67 ( online [PDF]).
- ↑ GM Mitchell: Design and synthesis of a macrocyclic phospholipid . ( online ).
- ↑ Archaeal lipid . In: SBKB, PSI-Nature Structural Biology Knowledgebase . doi : 10.3942 / psi_sgkb / fm_2012_12 .