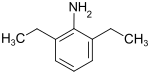
2,6-diethylaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,6-diethylaniline | ||||||||||||||||||
| other names |
2-amino-1,3-diethylbenzene |
||||||||||||||||||
| Molecular formula | C 10 H 15 N | ||||||||||||||||||
| Brief description |
light- and air-sensitive colorless to yellowish liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.24 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.96 g cm −3 |
||||||||||||||||||
| Melting point |
3 ° C |
||||||||||||||||||
| boiling point |
243 ° C |
||||||||||||||||||
| Vapor pressure |
0.03 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.545 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2,6-Diethylaniline is a chemical compound from the group of aromatic amino compounds ( amino group on the ring), more precisely the diethylanilines .
Extraction and presentation
2,6-Diethylaniline can be produced by Friedel-Crafts alkylation of aniline with ethene in the presence of aluminum chloride or mercury chloride.
properties
2,6-Diethylaniline is a colorless liquid (as a technical product, dark red liquid), which turns yellow when exposed to light and air. When heated, it decomposes, producing nitrogen oxides , carbon monoxide and carbon dioxide . The hydrochloride of the compound , which is insoluble in water, is formed with hydrogen chloride . The vapor pressure function results according to August according to ln (P) = A− (B / T) (P in Pa, T in K) with A = 27.83 and B = 7869 in the temperature range from 10 to 55 ° C. The enthalpy of vaporization at 25 ° C is 65.89 kJ mol −1 , the heat capacity c p 305.942 J K −1 mol −1 . The compound forms inflammable vapor-air mixtures at high temperatures. The compound has a flash point of 109 ° C. The ignition temperature is 460 ° C. The substance therefore falls into temperature class T1.
use
2,6-Diethylaniline is used as an intermediate in the manufacture of crop protection products (such as alachlor , butachlor , diafenthiuron ) and other derived chemical compounds. It also reappears as a metabolite of these compounds.
Web links
- PMG Bavin, JMW Scott: SOME CHEMISTRY OF 2,6-DIETHYLANILINE . In: Canadian Journal of Chemistry . 36 (9), 1958, pp. 1284-1288, doi : 10.1139 / v58-188 .
Individual evidence
- ↑ a b c d e f g h Entry on 2,6-diethylaniline in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c data sheet 2,6-diethylaniline from Sigma-Aldrich , accessed on February 9, 2010 ( PDF ).
- ↑ a b ( page no longer available , search in web archives: DEA (Albermarle) )
- ↑ Entry on 2,6-diethylaniline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 2,6-diethylaniline from AlfaAesar, accessed on February 5, 2010 ( PDF )(JavaScript required) . .
- ↑ Patent DE951501 : Process for the production of ring-alkylated aromatic amines. Registered on December 25, 1953 , published on October 31, 1956 , applicant: Bayer AG, inventor: Rudolf Stroh, Josef Ebersberger, Hans Haberland.
- ^ R. Stroh, J. Ebersberger, H. Haberland, W. Hahn: Newer methods of preparative organic chemistry II. 7. Alkylation of aromatic amines. In: Angew. Chem. 69, 1957, pp. 124-131, doi: 10.1002 / anie.19570690403 .
- ↑ a b S. P. Verevkin: thermochemical study of the ortho interactions in alkyl substituiertem anilines. In: J. Chem. Thermodyn. 32, 2000, pp. 247-259, doi: 10.1006 / jcht.1999.0587 .
- ↑ a b E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
