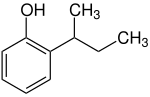
2- sec -butylphenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-sec-butylphenol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 14 O | |||||||||||||||
| Brief description |
colorless to yellowish liquid with a phenolic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 150.22 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.982 g cm −3 |
|||||||||||||||
| Melting point |
12 ° C |
|||||||||||||||
| boiling point |
226-228 ° C |
|||||||||||||||
| Vapor pressure |
0.08 hPa (20 ° C) |
|||||||||||||||
| solubility |
sparingly soluble in water (1.46 g l −1 at 20.1 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2- sec -Butylphenol is an aromatic compound from the group of alkylphenols .
Isomers
2- sec -Butylphenol has a stereocenter and is consequently a chiral compound of which there are two isomers , ( R ) -2- sec -Butylphenol and ( S ) -2- sec -Butylphenol.
Unless expressly stated otherwise in this text or in the scientific literature, "2- sec -Butylphenol" is the racemate, i.e. the 1: 1 mixture of ( R ) -2- sec -Butylphenol and ( S ) -2- sec -butylphenol.
| Isomers of 2-sec-butylphenol | ||
| Surname | ( S ) -2- sec -butylphenol | ( R ) -2- sec -butylphenol |
| other names | (+) - 2- sec -Butylphenol | (-) - 2- sec -butylphenol |
| Structural formula |

|

|
| CAS number | 159650-89-2 | 36383-18-3 |
| 89-72-5 (racemate) | ||
| EC number | - | - |
| 201-933-8 (racemate) | ||
| ECHA info card | - | - |
| 100.001.758 (racemate) | ||
| PubChem | 5325936 | 7000004 |
| 6984 (racemate) | ||
| Wikidata | Q28600794 | Q27115978 |
| Q20156241 (racemate) | ||
Extraction and presentation
2- sec -Butylphenol is industrially produced by a Friedel-Crafts alkylation of phenol with 1-butene (or 2-butene ) at temperatures of 250-300 ° C. and pressures of 35-80 bar on acidic gamma-aluminum oxide contacts as Catalyst made.
The catalyst is finely suspended in a discontinuous process . In the liquid phase, stirred tank reactors are used and phenol is used in an approximately four-fold excess. The selectivity for 2- sec- butylphenol reached 95%. Small amounts of 4 sec -butylphenol and sec -butylphenyl ether are also formed as by-products .
properties
2- sec -Butylphenol is a flammable, hardly inflammable, colorless to yellowish liquid with a phenol-like odor, which is sparingly soluble in water.
use
2- sec -Butylphenol is used as an intermediate in the manufacture of insecticides , acaricides , herbicides and other chemical compounds.
safety instructions
The vapors of 2- sec -butylphenol can form an explosive mixture with air ( flash point 112 ° C, ignition temperature 270 ° C).
Individual evidence
- ↑ a b c d e f g h i j k l m entry on 2-sec-butylphenol in the GESTIS substance database of the IFA , accessed on December 6, 2016(JavaScript required) .
- ↑ a b c Helmut Fiege, Heinz ‐ Werner Voges, Toshikazu Hamamoto, Sumio Umemura, Tadao Iwata, Hisaya Miki, Yasuhiro Fujita, Hans ‐ Josef Buysch, Dorothea Garbe, Wilfried Paulus: Phenol Derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., June 15, 2000, p. 17, doi : 10.1002 / 14356007.a19_313 .


