Azodicarboxylic acid diethyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Azodicarboxylic acid diethyl ester | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 N 2 O 4 | |||||||||||||||
| Brief description |
orange liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 174.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.11 g cm −3 |
|||||||||||||||
| boiling point |
211-217 ° C |
|||||||||||||||
| solubility |
miscible with dichloromethane , diethyl ether and toluene |
|||||||||||||||
| Refractive index |
1.4210 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diethyl azodicarboxylate , usually abbreviated as DEAD ( D i e thyl a zo d icarboxylate), is an important reagent for the Mitsunobu reaction , but it can be used in many ways.
Manufacturing
The preparation takes place via derivatives of hydrazine , which are dehydrated by suitable means . One possibility is oxidation using fuming nitric acid . The reaction also succeeds using chlorine as the oxidizing agent .
Chemical properties, safety
DEAD is toxic, shock and light sensitive and thermally unstable. The compound is explosive in terms of the Explosives Act and is assigned to substance group A there. In the steel sleeve test , the substance reacts extremely violently with a limit diameter of 20 mm. The impact sensitivity is only 4 years. In the lead block test , an expansion of 33 ml / 10 g is observed. Above 100 ° C, a strongly exothermic decomposition takes place with a heat of decomposition of −1466 kJ kg −1 or −255 kJ mol −1 . It is therefore usually available commercially in dissolved form, for example in toluene . As a pure substance, DEAD may not be shipped in the USA . Due to these safety risks, the use of DEAD decreased; it is mostly replaced by the more stable diisopropylazodicarboxylate (DIAD).
DEAD can explode during distillation. Appropriate safety precautions must be taken. Direct light sources should be shielded.
use
Mitsunobu reaction
The classic area of application of DEAD is the Mitsunobu reaction, which is used for the synthesis of esters , ethers , amines and thioethers from alcohols .
Enophile
Another area of application of DEAD is its use as an enophile, for example in ene reactions .
Dienophile
The use as a dienophile is also described in the literature. For example, the synthesis of bicyclo [2.1.0] pentane was achieved starting from cyclopentadiene and DEAD.
Michael acceptor
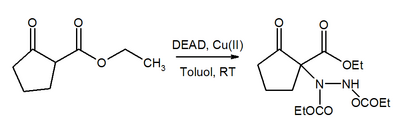
The azo group in DEAD is also a Michael acceptor . In the presence of copper catalysts added DEAD of β-keto esters to the corresponding hydrazine derivatives.
Similarly , Cu (II) also catalyzes the substitution of boronic acid esters in almost quantitative yield.
Synthesis of pyrrazoline derivatives
DEAD can also be used for the synthesis of heterocycles . Thus Pyrrazolin derivatives by condensation of α, β- unsaturated ketones are obtained .:
literature
- Clayden, Greeves, Warren & Wothers: Organic Chemistry. Oxford University Press, August 2004, ISBN 0-19-850346-6
- O. Mitsunobu, M. Wada, T. Sano: Stereospecific and stereoselective reactions. I. Preparation of amines from alcohols in J. Am. Chem. Soc. 94 (1972) 679-680, doi : 10.1021 / ja00757a085 .
- RFC Brown, WR Jackson, TD McCarthy: Potential routes to flavan-3-ols, part 2: The Mitsunobu reactions of para-oxygenated benzylic alcohols in Tetrahedron 50 (1994) 5469-5488, doi : 10.1016 / S0040-4020 (01) 80702-X . (Method development)
- O. Mitsunobu: The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products in Synthesis 1981, 1-28, doi : 10.1055 / s-1981-29317 . (Overview)
Individual evidence
- ↑ a b Entry on diethyl diazenedicarboxylate. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d e data sheet Diethyl azodicarboxylate, 97% from AlfaAesar, accessed on December 26, 2019 ( PDF )(JavaScript required) .
- ↑ Norman Rabjohn: Ethyl Azodicarboxylate In: Organic Syntheses . 28, 1948, p. 58, doi : 10.15227 / orgsyn.028.0058 ; Coll. Vol. 3, 1955, p. 375 ( PDF ).
- ↑ a b J. C. Kauer: Ethyl Azodicarboxylate. In: Organic Syntheses , Coll. Vol. 4 (1963), p. 411, doi : 10.1002 / 0471264180.os900.12 ( PDF ).
- ↑ Announcement of the new findings made by the BAM since 1987 in accordance with § 2 SprengG - notification of assessment 402 of February 16, 2001 pdf link .
- ↑ a b c d Berger, A .; Wehrstedt, KD: Azodicarboxylates: Explosive properties and DSC measurements in J. Loss Prev. Proc. Ind. 23 (2010) 734-739, doi : 10.1016 / j.jlp.2010.06.019 .
- ^ Lehmann, Neumann: En reaction Uni Hannover ( Memento from June 11, 2007 in the Internet Archive )
- ^ PG Gassman and KT Mansfield, Organic Syntheses Coll. Vol. 5 1973 , 96.
- ↑ Comelles, J .; Moreno-Mañas, M .; Perez, E .; Roglans, A .; Sebastián, RM; Vallribera, A .: Ionic and Covalent Copper (II) -Based Catalysts for Michael Additions. The Mechanism in J. Org. Chem. 69 (2004) 6834-6842, doi : 10.1021 / jo049373z .
- ↑ Uemura, T .; Chatani, N .: Copper Salt Catalyzed Addition of Arylboronic Acids to Azodicarboxylates in J. Org. Chem. 70 (2005) 8631-8634, doi : 10.1021 / jo051387x .
- ↑ Vijay, N .; Smith, CM; Akkattu, TB; Eringathodi, S .: A Novel Reaction of the “Huisgen Zwitterion” with Chalcones and Dienones: An Efficient Strategy for the Synthesis of Pyrazoline and Pyrazolopyridazine Derivatives in Angew. Chem. Int. Ed. 46 (2007) 2070-2073, doi : 10.1002 / anie.200604025 .