Chlorantraniliprole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
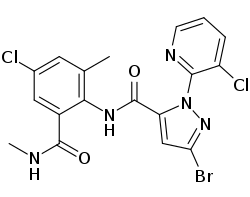
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chlorantraniliprole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 14 BrCl 2 N 5 O 2 | ||||||||||||||||||
| Brief description |
light yellow, finely crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 483.15 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.51 g · cm -3 |
||||||||||||||||||
| Melting point |
209 ° C |
||||||||||||||||||
| boiling point |
decomposes at 330 ° C |
||||||||||||||||||
| Vapor pressure |
6.3 · 10 –12 Pa (at 25 ° C) |
||||||||||||||||||
| solubility |
very sparingly soluble in water (0.88 mg · l –1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chlorantraniliprole is a synthetic insecticide from the group of active ingredients called ryanodine receptor modulators.
It was introduced by the DuPont Crop Protection company in 2007.
Mode of action
In its mode of action, chlorantraniliprole is comparable to other ryanodine receptor modulators such as flubendiamide or the structurally related cyantraniliprole . It interacts with the ryanodine receptor , which as calcium channels the Ca 2+ -transport in muscle control. The receptor is activated by chlorantraniliprole, which releases calcium ions in the cell. This leads to loss of control of muscle movements, resulting in paralysis and death .
Areas of application
Chlorantraniliprole is used successfully in arable and fruit growing. It is mainly used as a spray. It is particularly effective against biting and sucking pests such as the codling moth potato beetle and the crossed grape moth .
toxicology
In animal experiments it could be proven that chlorantraniliprole a low toxicity for humans and mammals as well as birds and fish has. In contrast, the active ingredient is highly toxic to insects and aquatic invertebrates . The European Food Safety Authority (EFSA) gives an ADI of 1.56 mg / kg.
Despite its classification as slightly toxic to humans, one case of poisoning by chlorantraniliprole has been reported in India .
Analytics
Chlorantraniliprole can be reliably detected and quantified using liquid and gas chromatographic methods. A mass spectrometer can be used for identification after the chromatographic separation .
Admission
Chlorantraniliprole is approved in most EU countries. This is also the case in Germany, Austria and Switzerland, where it is used, for example, in the Coragen plant protection product.
Trade names
- Coragen
- Rynaxypyr
- Altacor
- Prevathon
Individual evidence
- ↑ a b c data sheet Chlorantraniliprole from Sigma-Aldrich , accessed on May 17, 2018 ( PDF ).
- ↑ a b c d e f Entry on Chlorantraniliprole in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed May 17, 2018.
- ↑ a b c d Conclusion on the peer review of the pesticide risk assessment of the active substance chlorantraniliprole . In: EFSA Journal . tape 11 , no. 6 , June 2013, ISSN 1831-4732 , p. 3143 , doi : 10.2903 / j.efsa.2013.3143 .
- ↑ Suzhen Qi, John E. Casida: Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor . In: Pesticide Biochemistry and Physiology . tape 107 , no. 3 , November 2013, ISSN 0048-3575 , p. 321–326 , doi : 10.1016 / j.pestbp.2013.09.004 .
- ↑ Andrea Bassi et al .: CHLORANTRANILIPROLE (DPX-E2Y45, RYNAXYPYR®, CORAGEN®), A NEW DIAMIDE INSECTICIDE FOR CONTROL OF CODLING MOTH (Cydia pomonella), COLORADO POTATO BEETLE (Leptinotarsa decemlineata. (PDF) In: Zbornik predavanj in referatov 9 slovenskega posvetovanja o varstvu rastlin z mednarodno udeležbo Nova Gorica. March 2009, accessed May 17, 2018 .
- ↑ Modification of the existing maximum residue levels for chlorantraniliprole in various crops . In: EFSA Journal . tape 13 , no. 9 , September 2015, p. 4216 , doi : 10.2903 / j.efsa.2015.4216 .
- ↑ VigneshKumar Chandiraseharan, AjayKumar Mishra, Nisha Jose, ThambuDavid Sudarsanam: Chlorantraniliprole: A novel insecticide poisoning in humans . In: Indian Journal of Critical Care Medicine . tape 20 , no. 12 , 2016, ISSN 0972-5229 , p. 742 , doi : 10.4103 / 0972-5229.195718 , PMID 28149035 , PMC 5225778 (free full text).
- ↑ Timo Schwarz, Timothy A. Snow, Christopher J. Santee, Christopher C. Mulligan, Thomas Class: QuEChERS Multiresidue Method Validation and Mass Spectrometric Assessment for the Novel Anthranilic Diamide Insecticides Chlorantraniliprole and Cyantraniliprole . In: Journal of Agricultural and Food Chemistry . tape 59 , no. 3 , February 2011, p. 814-821 , doi : 10.1021 / jf103468d .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Chlorantraniliprole in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on May 18, 2018.

