Tranexamic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
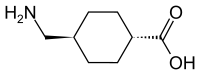
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tranexamic acid | |||||||||||||||||||||
| other names |
trans -4- (aminomethyl) cyclohexane-1-carboxylic acid (AMCHA) |
|||||||||||||||||||||
| Molecular formula | C 8 H 15 NO 2 | |||||||||||||||||||||
| Brief description |
beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Fibrinolysis inhibitors |
|||||||||||||||||||||
| Mechanism of action |
Lysine analog |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 157.21 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
386–392 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
| Pharmacological information | |||
|---|---|---|---|
| Mode of administration | peroral (po); intravenous (iv); local | ||
| Bioavailability | 30–50% after oral administration, unaffected by simultaneous food consumption. Across the placenta 100%. Plasma protein binding 3% (almost exclusively to plasmin). Breast milk 1%. | ||
| metabolism | minor in the liver; terminal half-life 2 hours, volume of distribution 9–12 L. | ||
| Interactions | Factor IX | Risk of thrombosis |
|
| excretion | urine | 95% as unchanged substance | |
| chair | - | ||
| Incompatibility | NN | - | |
| Clinical information | |||
| Indication (s) | children | Prevent or reduce bleeding in haemophilia (e.g. bleeding gums) | |
| Adults | Prevent or reduce bleeding in haemophilia (e.g. bleeding gums) | ||
| Side effects | Visual disturbances. Nausea, vomiting, diarrhea. Drop in blood pressure (with rapid intravenous administration). | ||
| Contraindication (s) | Renal insufficiency (dose adjustment required). Urinary tract bleeding (risk of obstruction). Existing thrombosis. | ||
| Admission status | |||
| Germany | United States | EU | |
| Approval date | DD.MM.YYYY | 12/30/1986 | DD.MM.YYYY |
| status | Pharmacy only. Prescription only. | ||
Tranexamic acid ( AMCHA or TXA ) is a substance used in medicine to inhibit the fibrinolysis system. The mechanism of action is based on the formation of a complex with plasminogen , whereby its binding to the fibrin surface is inhibited. This ultimately results in an inhibition of clot dissolution (fibrinolysis). It is therefore called an antifibrinolytic (fibrinolysis inhibitor).
Origin and manufacture
Tranexamic acid is a synthetic substance that is similar to the amino acid lysine . Like ε-aminocaproic acid and p- aminomethylbenzoic acid, it belongs to the group of so-called ε-aminocarboxylic acids .
Mechanism of action
Tranexamic acid blocks the formation of plasmin by inhibiting the proteolytic activity of the plasminogen activators. This hampers plasmin's ability to lyse fibrin. At a low dose, tranexamic acid acts as a competitive inhibitor of plasmin, at a high dose as a non-competitive inhibitor. All ε-aminocarboxylic acids act analogously.
Pharmacokinetics
Uptake and bioavailability
Tranexamic acid is 30–50% bioavailable after oral administration. The volume of distribution is 9–12 L. The half-life is 2 hours.
Metabolism
Tranexamic acid is only very slightly metabolized in the liver. Carboxylic acid (1% of the administered dose) and the acetylated form of tranexamic acid (0.1% of the administered dose) were found in the urine as metabolic products.
excretion
95% of it is excreted via the kidneys and urinary tract (renal elimination). Due to the almost exclusively renal elimination of the substance, the dose must be reduced in renal insufficiency , especially after prolonged use, so that no accumulation of tranexamic acid in the plasma occurs. Depending on the creatinine in the serum, the number of single doses per day is reduced.
Interactions
An increased risk of thrombosis is observed when tranexamic acid and factor IX are administered together .
application areas
- Used to prevent or relieve bleeding during tooth extraction or bleeding gums in haemophilia .
- Generalized and / or local hyperfibrinolysis (increased fibrinolysis). Reduction of hyperfibrinolysis, caused either by excess plasmin (hyperplasminemia) or as a result of thrombolytic treatment with, for example, streptokinase . Increased local fibrinolysis can occur with prostate and urinary tract operations, with recurrent bleeding of the gastrointestinal tract , with ulcerative colitis , with essential or IUD- induced hypermenorrhea (increased menstrual bleeding), with nosebleeds and after tooth extraction in patients with coagulopathies ( coagulopathies ). Tranexamic acid is also used in operations with extracorporeal circulation (heart-lung machine) and is also used in acute craniocerebral trauma .
- Tranexamic acid is also used as an antidote to block fibrinolytics such as streptokinase. As an alternative, the proteinase inhibitor (antiplasmin) aprotinin was also given , which was withdrawn from the market in 2007 because of its thrombogenic effect.
- Hereditary angioedema (HAE).
Side effects
- Allergic reactions occur both systemically (all over the body) and in the form of rashes.
- Tranexamic acid can lead to the formation or multiplication of thromboses , especially in patients with a congenital or acquired tendency to thrombosis (thrombophilia) . Thrombosis can subsequently lead to embolism (pulmonary embolism, stroke).
- Atrial fibrillation with an increased risk of stroke.
- Tranexamic acid can cause vision problems in people. Damage to the retina has been described in animal experiments.
Contraindications (contraindications)
- Lactation . Tranexamic acid is excreted in breast milk (in very low concentrations of about 1% of the plasma concentration).
- Bleeding in the urinary tract . The use of tranexamic acid can cause blockages of the ureters with subsequent urine congestion.
- Thrombosis . Thromboses (pre-existing) are promoted when tranexamic acid is administered.
- Sepsis and DIC (disseminated intravascular coagulation).
Dosage forms and strengths
- 1 film-coated tablet contains 500 mg tranexamic acid.
- 1 ampoule with 5 ml contains 500 mg tranexamic acid.
- 1 ampoule with 10 ml contains 1000 mg tranexamic acid.
- 1 effervescent tablet contains 1000 mg tranexamic acid.
Trade names
Cyklokapron , manufacturer: MEDA Pharma ; Quixil manufacturer: OMRIX biopharmaceuticals
Web links
- Deutsche Gesellschaft für Kardiotechnik eV: Is tranexamic acid a cost-effective alternative to aprotinin as an antifibrinolytic in open heart surgery using the heart-lung machine
- dosing.de: Tranexamic acid
- US package insert from Cyclokapron (PDF; 552 kB). Status 1999. Freely accessible.
Individual evidence
- ↑ a b c d data sheet trans-4- (aminomethyl) cyclohexanecarboxylic acid, 97% from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ a b c Entry on tranexamic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ Nicola Siegmund-Schultze: Acute traumatic brain injury. Tranexamic acid, infused early, reduces injury-related mortality. In: Deutsches Ärzteblatt. Volume 116, Issue 51 f., 23 December 2019, p. B 1974 f.
