Diimine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
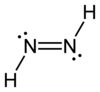
|
||||||||||
| General | ||||||||||
| Surname | Diimine | |||||||||
| other names |
|
|||||||||
| Molecular formula | H 2 N 2 | |||||||||
| Brief description |
bright yellow, metastable solid below −180 ° C |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 30.03 g · mol -1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Diimine (also diazene , diimide , Azowasserstoff ) is an inorganic compound , forming the basic structure of the organic azo compounds . It is unstable at room temperature . Pure, solid diimine, metastable below −180 ° C, has a bright yellow color. The compound cannot be sublimed without decomposition , is very sensitive to light and disproportionately disintegrates into nitrogen and hydrazine.
Extraction and presentation
Diimin can be obtained in the following ways:
- by dehydration of hydrazine (e.g. by exposure to microwaves )
- by converting azo compounds (e.g. by protolysis of azodicarbonate )
- by hydrogenation of nitrogen
Isomerism
The compound occurs in three isomeric forms, a trans ( 1 ), a cis ( 2 ) and an iso form ( 3 ).
history
The substance was isolated for the first time in 1972 by Nils Wiberg and co-workers. Thermolysis of sodium tosylhydrazide was carried out in vacuo at 60 ° C. The tosyl hydrazide disintegrated into diimine and sodium toluenesulfinate with 80% yield. The diimine was deposited as a bright yellow coating at −196 ° C on a cold finger . The detection in the gas was carried out by mass spectrometry . The preparation was also successful with other alkali metal tosylhydrazides, but in lower yield.
Diazenes, Triazenes, Tetracenes
The diazenes in which the hydrogen atoms of the diimine are replaced by alkyl or aryl groups are formally derived from the diimine . However, they are commonly referred to as azo compounds . Diazenes can in principle be in an ( E ) or a ( Z ) form, of which the former is the energetically more favorable one (see cis-trans isomerism ).
Also can formally triazenes and tetrazenes derived from diimine. A stable derivative of example, the triazene is diazoaminobenzene . A tetrazene derivative is obtained by oxidation of phenylhydrazine in the form of the yellow 1,1,4,4-tetraphenyl-2-tetrazene.
Diazenyl residue
In the IUPAC - nomenclature one can azo bridge as diazenyl - rest are called.
Inorganic derivatives
A derivative of diimine is hypo-nitrous acid (diazenediol). Another derivative is the difluorodiimine N 2 F 2 ( difluorodiazine ). The azo acids (diazenedicarboxylic acid) or their stable amides and esters (for example diethyl azodicarboxylate ) also belong to the derivatives. Azosilanes, such as trans-bis (trimethyl-silyl) diimine, are also known:
Diimine complexes
Are known, for example, polynuclear carbonyl - complexes with chromium and cyclopentadienyl complexes with manganese, prepared by oxidation from the corresponding hydrazine can be prepared complexes. In contrast to pure diimine, these diimine complexes are relatively stable in the solid state, but are also highly reactive in solutions.
These two complexes were the first compounds in which diimine could be fixed and characterized.
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 672.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Nils Wiberg , Heinz Bachhuber, Gerd Fischer: Isolation of Diimin, Angewandte Chemie , 84th year 1972, No. 18, p. 889, doi : 10.1002 / anie.19720841808
swell
- Dieter Sellmann: Diimin and its derivatives . In: Chemistry in Our Time . tape 7 , no. 6 , 1973, p. 163–170 , doi : 10.1002 / ciuz.19730070602 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , pp. 671-675.
- Entry on Diimin. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.




