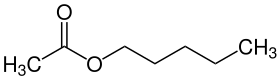
Acetic acid n -pentyl ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Acetic acid n -pentyl ester | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 7 H 14 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a pear-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 130.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.88 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−71 ° C |
||||||||||||||||||
| boiling point |
149 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4030 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acetic acid n- pentyl ester (according to IUPAC nomenclature : pentyl acetate , systematically also pentyl ethanoate ) is an organic-chemical compound from the group of carboxylic acid esters .
Extraction and presentation
Acetic acid n -pentyl ester is produced on an industrial scale by the acid-catalyzed esterification of acetic acid with n- pentanol .
According to the principle of Le Chatelier , separation of the water produced or the removal of the ester shifts the equilibrium to the product side (see also the law of mass action ).
properties
Physical Properties
Acetic acid n -pentyl ester has a relative gas density of 4.49 (density ratio to dry air at the same temperature and pressure) and a relative density of the vapor-air mixture of 1.02 (density ratio to dry air at 20 ° C and normal pressure ).
Chemical properties
Acetic acid n -pentyl ester is a flammable liquid from the group of carboxylic acid esters . The vapors can form explosive mixtures with air when the substance is heated above its flash point . This is possible even at higher ambient temperatures. It is also sparingly soluble in water (10 g / l at 20 ° C) and lighter than water. Acetic acid n- pentyl ester is of medium or low volatility.
use
Acetic acid n -pentyl ester is an important solvent for the paint industry . It has a high dissolving power for numerous natural and synthetic resins . That is why it is mostly used to dissolve or dilute acrylates or cellulose derivatives . It is also used as a solvent for automotive refinish paints based on polyurethane resins, nitro emulsion paints and special paints. Pentyl acetate is also used as an additive in cleaning agents or as an extraction agent for substances from aqueous systems. In the past, the Hefner candle was used as a light standard, using amyl acetate (= acetic acid n- pentyl ester) or a mixture of pentyl ester of acetic acid as fuel .
safety instructions
n -Pentyl acetate is mainly absorbed through the airways . Ingestion or ingestion can cause irritation to the respiratory tract, skin and eyes. In addition, there is a risk of central nervous system disruption at high concentrations . Chronic consequences of too long exposure can be skin changes and irritation to the mucous membranes . The explosion range is between 1.0% by volume (54 g / cm 3 ) as the lower explosion limit (LEL) and 7.5% by volume (405 g / cm 3 ) as the upper explosion limit (UEL). The maximum explosion pressure was determined to be 8.4 bar. The ignition temperature is 350 ° C. The substance therefore falls into temperature class T2. With a flash point of 41 ° C, n- pentyl acetate is considered to be relatively flame-retardant.
See also
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on pentyl acetate in the GESTIS substance database of the IFA , accessed on November 27, 2018 (JavaScript required)
- ↑ a b Pentyl Acetate - solvent for numerous synthetic and natural resins. BASF, accessed November 27, 2018 .
- ↑ Data sheet n-Pentyl acetate, 99% from AlfaAesar, accessed on January 11, 2020 ( PDF )(JavaScript required) .
- ↑ Entry on pentyl acetate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 30, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on n-amyl acetate in the Hazardous Substances Data Bank , accessed December 27, 2018.
- ↑ H. Lux: The modern lighting system , accessed on November 28, 2018.

