Triphenyl tin chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
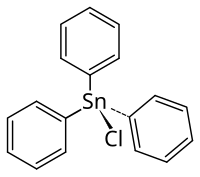
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenyl tin chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 15 ClSn | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 385.47 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
108 ° C |
|||||||||||||||
| boiling point |
240 ° C, at 18 hPa |
|||||||||||||||
| Vapor pressure |
7.3 10 −6 hPa (25 ° C) |
|||||||||||||||
| solubility |
1 mg l −1 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triphenyltin chloride is a chemical compound from the group of organotin compounds .
Extraction and presentation
Triphenyltin chloride can be made by reacting tetraphenyltin with hydrogen chloride in chloroform :
As early as 1918, Erich Krause described the preparation of triphenyltin chloride via the reaction of triphenyltin hydroxide with hydrogen chloride :
Technically, it can be represented by comproportioning tetraphenyltin with tin tetrachloride or phenyltin trichloride ( Kocheshkov rearrangement ):
properties
The solubility of trialky tin and triaryl tin compounds in organic solvents is significantly greater than in water, since the tin-carbon bond has a larger covalent content. These hydrolyze only slowly in water.
Physical Properties
In the 13 C nuclear resonance spectrum (NMR), triphenyltin chloride shows the following signals:
| Sn – C 1 - | -C 2 | -C 3 | -C 4 | |
|---|---|---|---|---|
| ppm | 137.6 | 136.5 | 129.4 | 130.6 |
| J ( 13 C– 119 Sn) | 614 | 50.7 | 64.5 | 13.0 |
In the 119 Sn NMR it gives a signal at −44.4 ppm. The tin-carbon distance in crystal is 2.14 Å . In benzene it has a dipole moment of 3.46 Debye .
use
Triphenyltin chloride is the starting compound for a number of other triphenyltin compounds, such as triphenyltin hydroxide and triphenyltin acetate .
Triphenyltin hydride can be made by hydrogenating triphenyltin chloride with a reducing agent such as lithium aluminum hydride :
In the past, triphenyltin compounds , such as triphenyltin chloride, were used as biocides and as algicides and molluscicides in antifouling paints . Their use is now largely prohibited worldwide. In the EU , triphenyltin compounds have been banned in agriculture since 1998 and since 2006 may no longer be used as biocides.
safety instructions
When triphenyltin compounds are absorbed, they accumulate in the kidneys, liver, brain and heart and affect the central nervous system. Triphenyltin chloride is gradually broken down into di- or monophenyltin. Based on the analogy to the effect of triphenyltin hydroxide , a MAK value of 0.002 mg tin · m −3 was established for all phenyltin compounds .
Individual evidence
- ↑ a b c d e f g h data sheet Triphenyltin chloride from Sigma-Aldrich , accessed on June 26, 2015 ( PDF ).
- ↑ a b c d e f Phenyltin compounds [MAK Value Documentation in German language, 2010] . In: The MAK Collection for Occupational Health and Safety . January 31, 2012, doi : 10.1002 / 3527600418.mb240668verd0048 .
- ↑ a b Entry on fentin chloride in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Bodo Heyn, Bernd Hipler, Günter Kreisel, Heike Schreer, Dirk Walther: Inorganische Synthechemie: An integrated internship . Springer-Verlag, 2013, ISBN 978-3-642-75914-7 , p. 43 ( limited preview in Google Book search).
- ↑ Erich Krause: Simplified representation of triaryl tin halides. In: Reports of the German Chemical Society. 51, 1918, p. 912, doi: 10.1002 / cber.191805101112 .
- ↑ Alwyn George Davies: organotin chemistry, Volume 1 . Wiley-VCH Verlag GmbH & Co. KGaA, 2004, ISBN 3-527-31023-1 ( page 167 in the Google book search).
- ↑ KA Kozeschkow: studies on organometallic compounds, II Messaging .: The reaction between the fatty series organotin compounds and tin tetrahalides. . In: Reports of the German Chemical Society . tape 66 , no. 11 , November 8, 1933, pp. 1661-1665 , doi : 10.1002 / cber.19330661109 .
- ↑ GJM van der Kerk, JGA Luijten, JG Noltes: New results in organotin research . In: Angewandte Chemie . tape 70 , no. 10 , May 21, 1958, pp. 298-306 , doi : 10.1002 / anie.19580701004 .
- ↑ a b Cathrin Zeppek, Johann Pichler, Ana Torvisco, Michaela Flock, Frank Uhlig: Aryltin chlorides and hydrides: Preparation, detailed NMR studies and DFT calculations . In: Journal of Organometallic Chemistry . tape 740 , September 2013, p. 41-49 , doi : 10.1016 / j.jorganchem.2013.03.012 .
- ↑ Jörg Lorberth, Heinrich Nöth: Dipole moments of some organotin chlorides . In: Chemical Reports . tape 98 , no. 3 , March 1965, p. 969 , doi : 10.1002 / cber.19650980342 .
- ↑ Eberhard Amberger, Heinz P. Fritz, Cornelius G. Kreiter, Maria-Regina Kula: Spectroscopic Investigations on Organometallic Compounds, XXV. Proton magnetic resonance spectra of triphenylstannane, diphenylstannane and monophenylstannane . In: Chemical Reports . tape 96 , no. December 12 , 1963, p. 3270-3274 , doi : 10.1002 / cber.19630961224 .
- ^ Federal Environmental Specimen Bank : Triphenyltin , accessed on November 17, 2013.








