Common cup maid
| Common cup maid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Common goblet maiden ( Enallagma cyathigerum ), male |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Enallagma cyathigerum | ||||||||||||
| ( Charpentier , 1840) |
The common cup dam ( Enallagma cyathigerum ) is a species of dragonfly from the family of the slender dragonfly (Coenagrionidae). It is relatively undemanding in its choice of habitat and colonizes a variety of different types of water - slow-flowing streams, ponds and ponds with open water surfaces as well as bog lakes - and is therefore one of the most widespread and most common dragonflies in Europe. It is classified as safe by the IUCN . The large distribution area extends over the whole of Europe and large parts of Asia, where populations with slightly different characteristics are formed - very dark colored males with extensive black markings. The common goblet maiden resembles different species of the azure maiden ( Coenagrion ) and is therefore also called goblet azure maiden in older literature .
The males of the typical, medium-sized slender dragonfly have a light blue basic color with black markings, the females appear in blue or brownish-green color variants. The adults are capable of a temperature-dependent physiological color change in which the body color is darkened to a brownish color. Like all dragonfly species, the common mugwort lives predatory as both larva and imago and is dependent on water to lay eggs for the aquatic larvae to develop. This can also take place under water.
features
Characteristics of the adults
The common cup damsel is a typical slender dragonfly with a body length of 29–36 mm and a wingspan of 40–45 mm. The male adults have a light blue basic color with black markings on the segment ends of the abdomen . In the middle of the upper side of the front body ( thorax ) there is a broader black median stripe, which is divided - rarely in males, more often in females - by a fine, light median suture, but the characteristic can be very weak or completely absent. The central stripe is flanked on both sides by the light blue ante-humeral stripes, these are at least as wide as the subsequent black humeral stripes. On the light blue sides of the thorax underneath there are two shorter black lines, the upper of the two is only very rudimentary or not at all, so that it is usually not perceptible without a closer look.
On the second segment of the abdomen, the eponymous drawing can be found in the form of a stemmed cup - sometimes this figure is also described as mushroom-shaped. However, it is quite variable and therefore cannot be used for determination alone. The drawing of abdominal segments three to five is similar, but more extensive and extends down the sides of the segment. On the sixth and seventh segment, the drawing then takes up almost the entire area. The eighth and ninth abdominal segments of the male adults are all blue again and thus form a striking "bottom light".
The females are more powerfully built; the black, lancet-like, pointed drawing is more extensive than that of the males. In front of the ovary, on the eighth abdominal segment, there is a protruding thorn. As is not uncommon in the subfamily of the Ischnurinae , the females come in different color variants. There are androchrome, like the males colored females, and greenish or light brownish-gray forms. Of the latter, it is not yet clear whether it is possibly the youth form of the green morph. The frequency distribution of the various color morphs varies greatly from region to region.
Shortly after the transformation to the imago, the dragonflies are still without a drawing - this only develops within the next few hours. The typical blue color is also only formed during the ripening in the following days. This color is not bound to pigments, but is formed by the refraction of light on a suspension of microscopic particles ( Tyndall effect ) in front of a black pigment layer within the epidermal cells and perceived from the outside through the transparent cuticle . As a result, Enallagma cyathigerum is able to undergo a physiological color change, in which pigment migration in the epidermal cells can darken the body color. Since this effect only occurs at low outside temperatures, it probably serves to warm up more quickly due to the higher absorption of solar radiation in the dark color phase.
Similar species

The common goblet maiden resembles different species of the azure maiden ( Coenagrion ) and is therefore also called goblet azure maiden in many older identification books. However, it belongs to the genus of the cup maiden ( Enallagma ), so that the name "common cup maiden", which correctly describes the genus, is usually used today. Especially the females of both genera can easily be confused by their very similar drawings. A definite distinguishing feature is, in addition to the protruding spike in front of the ovary, the drawing of the thorax sides. In both males and females of Enallagma cyathigerum , this area is not marked except for a small, narrow line; in the azure maidens and also in the similarly similar cup maiden ( Erythromma lindenii ) two clearly pronounced lines can always be seen here. Brown females can be reminiscent of the females of the winter dragonflies ( Sympecma ).
Characteristics of the larvae
The length of the larvae is 14–18 mm, that of the gill leaflets an additional 6–7 mm. The color of the entire body is very variable and ranges from green to dark brown, with specimens from heather bogs being particularly dark in color. The antennae of the head are divided into six, sometimes seven, segments. The femora are banded once shortly before the joint to the tibia , the tibiae themselves are not shown. At the end of the body there are the pointed gill leaves, which are large compared to larvae of other species and, especially at the base, wide. These can be drawn with up to three dark transverse bands in the distal half of the gill leaves. However, this pattern is highly variable; the bands vary in intensity between individuals and can also be completely absent. On the first up to two thirds of the total length, the flattened gill leaves are lined with small bristles at the edges. In some similar species, a notch marks the transition to the unlined area; this is missing in E. cyathigerum . The gill leaflets, conspicuously waved, can be used as a threatening gesture against conspecifics. Usually the very active larvae are peaceful with each other. Mainly water flea and mosquito larvae are captured ; overwintering takes place in the penultimate or one of the larval stages that precede it. The larvae of the common dragonfly are similar to the larvae of other dragonflies, but are much more transparent. A safe identification feature is a small secondary bristle at the base of the last catching bristle of the catching mask. The gill leaflets are significantly less pointed than those of the pitch dragonflies and the back of the head lacks any markings of spots, as is typical for the larvae of horseshoe and bat damselfly .
distribution and habitat
distribution
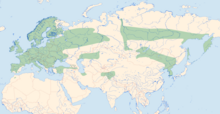
The Palearctic distribution area of Enallagma cyathigerum extends from the High Atlas to Kamchatka and north over the tundra zone and the Arctic Circle to the Arctic Ocean . Only recently has it been recognized that the North American population , which has also been assigned to E. cyathigerum , is to be regarded as a separate species; it has since been run as the Enallagma annexum . Even if the common cup maiden has lost its circumpolar status, it is one of the most widespread dragonfly species. The species is mostly common in Central Europe, but restricted to mountainous areas in the Mediterranean region.
Geographical variation
Especially in the north and east of the range, there are very dark colored males, which do not correspond in all characteristics to those of the Central European populations. With them, the drawing of the abdomen is significantly more extensive, the laterally elongated stripes are reminiscent of the Siberian azure virgin ( Coenagrion hylas ), the ante-humeral stripes are narrowed or even broken. Additional dark lines may be found on the chest pages without drawings in the case of the Central European representatives. The northern and southern populations in Siberia are clearly distinguishable, but the characteristics vary along a ridge from north to south and appear in the temperate zones of Russia so mixed that a distinction is difficult, if not impossible.
This morphological variability led to a number of incorrect species descriptions. The status of some taxa as an independent species or subspecies of the common mugwort - especially Enallagma deserti and Enallagma risi - is viewed differently in the literature.
A study from 2004, in which features more than 1,500 Siberian Enallagma were examined -Exemplaren, comes to the conclusion that Enallagma deserti , E. linearized , E. strouhali and E. nigrolineata as synonyms are to be considered the polymorphic commons Blue Damselfly. According to this study, only E. c. risi recognized as a subspecies. Another study from 2002, which examined six populations of the Enallagma cyathigerum complex from North Africa, Europe, West and Central Asia using DNA analysis and electron microscopic examination of the male abdominal appendages , also came to the conclusion that the taxa E. c . deserti and E. c. risi are to be placed as subspecies of the common cup maiden, even if the males can be clearly distinguished by the shape of the upper abdominal appendages. In addition, the nominate form hybridizes with E. c. deserti , as also with E. c. risi . It is concluded from this that they have only recently diverged.
Other sources attribute species status to Enallagma risi and Enallagma deserti due to the clear morphological differences; Another study from Siberia in 2010 shows that E. cyathigerum and E. deserti occur together in the investigated area on the Vasyugan plain , but do not colonize the same habitats , from which a species status in addition to the morphological and recently investigated molecular differences for both taxa is derived. In a study that created a cladogram for the Enallagma species of the entire Holarctic using the mitochondrial DNA of mature male adults , the adjacent taxa Enallagma cyathigerum , E. deserti and E. risi are assigned species rank and they are assigned the Japanese species Enallagma circatum combined into a palaearctic clade .
It is noticeable that the genus in Europe and the continental part of the Palearctic, which counts over forty species worldwide, is only represented by the common mugwort - which came here as a phylogenetically young species only a short time ago - and their close relatives.
habitat
The common mugwort colonizes a variety of different types of water, preferably still water with open water surface or slowly flowing water, but is otherwise undemanding. As a pioneer species, it can often be found on newly created lakes, pits and ponds. Silting up waters are avoided, and Enallagma cyathigerum is rarer even in waters with high fish populations - probably because of the high feeding pressure. In the north of Germany, moor waters are primarily populated, sometimes in high density. Enallagma cyathigerum is widespread and common in much of Europe and northern Asia. It is one of the most common dragonflies in the northern part of the range.
Endangerment and legal position
The International Union for Conservation of Nature and Natural Resources (IUCN) classifies the common cup virgin as harmless ( least concern ). In Germany it is not under any explicit protection, but like all dragonflies it is specially protected according to Appendix 1 of the Federal Species Protection Ordinance. According to the Red List of Endangered Dragonflies in Switzerland, it is classified as not endangered. A future threat could arise from water pollution and the increasing destruction of habitats, even if this is not expected to have any noticeable impact on the overall population.
Way of life
The main flight time for adults is between June and August. In contrast to other small dragonflies, the common mermaid often flies over the open water surface and uses the sparse vegetation, which protrudes only a few centimeters from the water, as a seat guard. The males keep their bodies almost horizontal and can often be identified from a distance due to this specificity. Like the majority of the dragonflies, E. cyathigerum is a stalker who flies horizontally at potential prey, takes it with its legs and returns with it to the waiting room to consume it there. The range of prey includes small and very small insects such as biting and black flies , but the species has no specific requirements and instead eats anything that can overwhelm it. Becherjungfern also undertake search flights for the targeted acquisition of prey in the vegetation at the edge of the water. Sedentary animals, such as aphids , are also picked up or prey is specifically picked from web spiders without coming into contact with the spider web.
Reproduction
From mid-May onwards, the mature females seek the waters for breeding, where they are expected by the males. Often a male succeeds in matching a female beforehand, so that the pair reaches the breeding waters as a tandem. Females appearing alone are grabbed by the males with the abdominal appendages on the prothorax ; the abdominal appendages and the shape of the prothorax are matched to each other in a species-specific manner and fit together perfectly. The pair then flies a further distance in a zigzag fashion, being pursued by other males who have so far been unsuccessful. These try to separate the tandem in order to be able to grab the female themselves. The copulation takes place sitting in the riparian vegetation and can last between ten and sixty minutes. The dragonflies form a mating wheel and curve their abdomen back so that the copulatory organs can touch and anchor one another. Before that, the male fills his seminal vesicle and leads the seminal discharge duct at the end of the abdomen to his copulation organ further forward.
During copulation, the male clears any sperm from other males from previous copulations before the sperm transfer with the secondary copulation organ. In addition, it has structures at the tip of the penis with which it can grab foreign sperm and remove it from the genital tract of the female. Since the eggs are only fertilized immediately before they are laid, it gives its own sperm a more favorable position for fertilization and thus a reproductive advantage for itself.
Egg laying
After mating, the couple flies out onto the open water surface in a tandem flight to begin laying eggs. It settles on plants that protrude briefly above the surface of the water, where the female drills the eggs into living or dead plant tissue with her ovipositor and lays up to eight eggs per minute. The male remains initially coupled to the female, which regularly dives backwards under water while laying eggs. Once the female is completely submerged, the male disconnects. The female then turns around and continues to climb upside down under the water as she bores the eggs into the aquatic vegetation in an irregular zigzag line. It cleverly changes over to other plants and can remain under water for up to 90 minutes. This is the longest dive time ever recorded for a dragonfly. The animal is protected by the physical gill , a fine layer of air that surrounds the submerged dragonfly and allows it to breathe underwater. Used oxygen is replaced in the air layer by diffusion from the water, while the carbon dioxide produced diffuses back. If necessary, this exchange of air can be increased by rolling movements around the longitudinal axis of the body.
Under water, the females are exposed to high feeding pressure from predators, but also to the risk of being held back by the surface tension of the water after surfacing and not being able to fly up. The females of Enallagma cyathigerum therefore try to lay down their entire egg supply as possible in a single dive. The male usually awaits the return of his partner at the surface of the water and tries to prevent the re-mating with another male. After the female emerges, she will pair again to bring her to another egg-laying site. If it is not possible to pull the female out of the water, both try to reach the next emers substrate. The male pulls his partner through the water, supported by the latter by flapping his forewings. The speed achieved in this way can be up to ten centimeters per second.
Sometimes the male will leave the female's diving site directly to try to mate with another female. The resurfaced female draws attention to herself with characteristic movements of the abdomen, if she cannot start out of the water on her own, so that she is usually found after a short time by one of the males flying in high density.
Larval development
The larvae hatch two to three weeks after the eggs are laid. The larvae go through ten to twelve molting stages before they are transformed into imago, in Central Europe usually within a year. In cool mountain locations, the development can take up to four years. The larva overwinters in one of the last molting stages.
The hatching period is between late April and early September, with the highest hatching rate in June. In contrast to many other dragonfly larvae that migrate to the shallow bank to hatch, Enallagma cyathigerum mostly uses vertical stalks of open water vegetation. The hatch then occurs only a few centimeters above the water surface.
Systematics
The common cup maiden was first described in 1840 by Toussaint von Charpentier as Agrion cyathigerum in his work Libellulineae Europaeae . It is placed within the slender vials in the genus of cup maids ( Enallagma ), which was also created in 1840 by Toussaint von Charpentier. Enallagma cyathigerum is a type of the genus.
The scientific name refers to "Enallagma" ( Greek for interchanging ) and was originally chosen by Charpentier for all blue and difficult to distinguish slender vials. The specific epithet cyathigerum ( lat. ) Is formed from "cyathus" (Greek loan word cup ) and -ger (um) (lat. Bearing ).
literature
- Klaus Sternberg, Rainer Buchwald (ed.): The dragonflies of Baden-Württemberg. Volume 1: General part, dragonflies (Zygoptera). Verlag Eugen Ulmer, Stuttgart 1999, ISBN 3-8001-3508-6 .
- Klaas-Douwe B. Dijkstra: Field Guide to the Dragonflies of Britain and Europe. British Wildlife Publishing, Gillingham 2006, ISBN 0-953139948 .
- Heiko Bellmann: The Kosmos dragonfly guide. Determine the species of Central Europe with certainty. Franckh Kosmos Verlag, Stuttgart 2007, ISBN 978-3-440-10616-7 .
- Gerhard Jurzitza: The Kosmos dragonfly guide . The species of Central and Southern Europe. Franckh Kosmos Verlag, Stuttgart 2000, ISBN 3-440-08402-7 .
- Steve Cham: Field Guide to the larvae and exuviae of British Dragonflies. Shrewsbury, The British Dragonfly Society, Peterborough 2012, ISBN 978-0-9556471-2-3 .
Web links
- Picture of a clearly dark colored male from Yakutia on naturfotografen-forum.de
Individual evidence
- ^ Jill Silsby: Subfamily Ischnurinae (Blue-tailed Damselflies). In Dragonflies of the World. Smithsonian, Washington 2001, ISBN 1-560-98959-9 , pp. 110-112.
- ↑ a b c d e f g h i K. Sternberg, F.-J. Schiel: Enallagma cyathigerum. In Sternberg, Buchwald: The dragonflies of Baden-Württemberg. Volume 1 , p. 300 ff.
- ↑ K. Sternberg: Construction and function of the dragonfly body - body color. In Sternberg, Buchwald: The dragonflies of Baden-Württemberg. Volume 1 , p. 91 ff.
- ↑ K. Sternberg: Thermoregulation. In Sternberg, Buchwald: The dragonflies of Baden-Württemberg. Volume 1 , p. 133 ff.
- ↑ a b c Steve Cham: Common Blue Damselfly Enallagma cyathigerum. In Field Guide to the larvae and exuviae of British Dragonflies , pp. 82-85.
- ↑ a b Heiko Bellmann: Becher-Azurjungfer. In The Cosmos Dragonfly Guide. Determine the species of Central Europe with certainty , Franckh-Kosmos-Verlag, Stuttgart 2007, ISBN 978-3-440-10616-7 , pp. 142-143.
- ↑ a b c Reinhard Jödicke: Enallagma cyathigerum (Charpentier, 1840). In Dijkstra: Field Guide to the Dragonflies of Britain and Europe , pp. 101-103.
- ↑ a b c d Enallagma cyathigerum in the IUCN Red List of Threatened Species 2010.4. Listed by: R. Dow, 2007. Retrieved August 22, 2013.
- ^ Dennis Paulson: Northern Bluet. In Dragonflies and Damselflies of the East , pp. 95-97.
- ↑ a b c d A. Yu. Haritonov: The composition and History of Siberian Odonate Fauna. Pp. 73-87. In BK Tyagi (ed.): Odonata. Biology of Dragonflies. Scientific Publishers (India), Jodhpur 2007, ISBN 978-81-7233-482-6 .
- ↑ B. Samrouai, PHH Weekers, HJ Dumont: The Enallagma of the western and central Palearctic (Zygoptera: Coenagrionidae). In: Odonatologica 31 (4), 2002, pp. 371-381. ISSN 0375-0183 .
- ↑ Asmus Schröter: The Odonata of Kyrgyzstan, part I - Critical nation checklist, annotated list of records and collected data of the summer half-years 2008 and 2009, International Dragonfly Fund - Report 28 (2010): 1-72.
- ↑ Reinhard Jödicke: Enallagma deserti (Selys, 1871). In Dijkstra: Field Guide to the Dragonflies of Britain and Europe , pp. 102-102.
- ↑ R. Bernard, OE Kosterin: biogeographical and ecological Description of the Odonata of eastern Vasyugan Plain, western Siberia, Russia In: Odonatologica 39 (1) of 2010.
- ↑ Julie Turgeon, Robby Stoks, Ryan A. Thum, Jonathan M. Brown, and Mark A. McPeek: Simultaneous Quaternary Radiations of Three Damselfly Clades across the Holarctic. The American Naturalist, 165, 4, 2005, pp. 78-107.
- ↑ Appendix 1 of the Federal Species Protection Ordinance
- ↑ Red list of endangered species in Switzerland: dragonflies . In: Swiss Federal Office for the Environment . Retrieved September 23, 2013.
- ↑ Christine Fischer: Enallagma cyathigerum and Ischnura elegans as kleptoparasites in spider webs (Odonata: Coenagrionidae). In Libellula, magazine of the Society of German-speaking Odonatologists eV 28 (3/4) 2009, ISSN 0723-6514 .
- ↑ A. Martens: Reproductive behavior of dragonflies - love in the wheel. In Sternberg, Buchwald: Die Libellen Baden-Württemberg. Volume 1 , p. 146 ff.
- ↑ A. Martens: Reproductive behavior of dragonflies - underwater egg deposit. In Sternberg, Buchwald: The dragonflies of Baden-Württemberg. Volume 1 , p. 156.