Glucono-1,5-lactone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
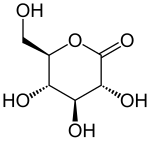
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | D - (+) - glucono-1,5-lactone | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 6 H 10 O 6 | |||||||||||||||||||||
| Brief description |
colorless, sweet-tasting solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 178.14 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
Decomposition: from 156–162 ° C |
|||||||||||||||||||||
| solubility |
good in water: 590 g l −1 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
D - (+) - glucono-1,5-lactone , also glucono-δ-lactone (GdL), is a lactone derivedfrom gluconic acid through intramolecular ester formation . Due to its structural and chemical properties, it is considered a carbohydrate in the broader sense.
properties
GdL forms sweet-tasting crystals. When it is put into water, it partially hydrolyzes to gluconic acid ; the more so, the warmer and / or more alkaline the environment.
use
It is used in detergents to prevent milk stone build-up.
It is used in the food industry as an acidity regulator and as an acid carrier for baking powder . It is generally approved in the EU as a food additive with the designation E 575 without maximum quantity restriction ( quantum satis ) for foodstuffs.
In 2009, researchers at the Massachusetts Institute of Technology discovered a biological method that uses glucono-1,5-lactone to switch off the special immune defense of termites, grasshoppers and cockroaches against bacteria and fungi that are deadly for animals. If the animals were exposed to this substance in the laboratory, they died of infections a few days later. Beneficial insects such as ants are spared because they have a different immune system. The use of glucono-1,5-lactone is also safe for humans and plants, making it an environmentally friendly alternative to conventional pesticides against insect pests.
Due to the structural similarity to the intermediate of glycogen phosphorylase (oxonium ion intermediate), glucono-1,5-lactone acts as an inhibitor of this enzyme . It is the first enzyme that ensures the rapid supply of energy for the metabolism during glycogen breakdown .
Individual evidence
- ↑ Entry on E 575: Glucono-delta-lactone in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on GLUCONOLACTONE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b Entry on d-gluconic acid-5-lactone. In: Römpp Online . Georg Thieme Verlag, accessed on June 8, 2014.
- ↑ a b c Entry on glucono-1,5-lactone in the GESTIS substance database of the IFA , accessed on August 4, 2016(JavaScript required) .
- ↑ Y. Pocker, Edmond Green: Hydrolysis of D -Glucono-δ-lactone. I. General Acid Base Catalysis, Solvent Deuterium Isotope Effects, and Transition State Characterization . In: J. Am. Chem. Soc. . 95, No. 1, 1973, pp. 113-19. doi : 10.1021 / ja00782a019 . PMID 4682891 .
- ↑ Blocking termites' defense mechanisms MIT News MIT News. Retrieved August 1, 2016.
- ^ Voet, Voet: Biochemistry, 3rd edition, p. 629.