Hyaluronic acid
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
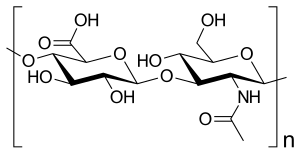 Disaccharide repeating unit of hyaluronic acid (-4GlcUA β 1-3GlcNAc β 1-) n |
|||||||||
| General | |||||||||
| Surname | Hyaluronic acid | ||||||||
| other names |
|
||||||||
| CAS number |
|
||||||||
| Monomers | D - glucuronic acid and N -acetyl- D -glucosamine | ||||||||
| Molecular formula of the repeating unit | C 14 H 21 O 11 N | ||||||||
| Molar mass of the repeating unit | 379.32 g mol −1 | ||||||||
| ATC code |
B06 AA03 , D03 AX05 , M09 AX01 , R01 AX09 , S01 KA01 , S01 KA51 |
||||||||
| Drug information | |||||||||
| Drug class |
Film maker |
||||||||
| properties | |||||||||
| Physical state |
firmly |
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Hyaluronic acid (according to the more recent nomenclature hyaluronan , abbreviation HA) is a glycosaminoglycan that is an important component of connective tissue and also plays a role in cell proliferation , cell migration and metastasis in some cancers . Hyaluronic acid was first discovered in the 1930s by the German physician Karl Meyer . The term hyaluronic acid is made up of the ancient Greek word ὕαλος hyalos ("glass") and uron , an abbreviation for uronic acids . Meyer discovered the substance while examining the vitreous .
Functions
Hyaluronic acid is a component of the extracellular matrix (ECM or ECM) of vertebrates. It is present in a variety of tissues as a long-chain, linear polysaccharide and fulfills many functions that are also based on its special chemical properties, such as the ability to bind a lot of water. It is not uncommon for the individual chains to reach a molar mass of several million atomic mass units .
Mechanical functions
Water storage
Hyaluronic acid has the ability to bind very large amounts of water relative to its mass (up to six liters of water per gram). The vitreous humor of the human eye z. B. consists of 98 percent water, which is bound to only two percent hyaluronic acid.
Pressure resistance
Water is hardly compressible, and this property remains valid even in tissue containing hyaluronic acid, in which a great deal of water can be bound. This generally applies to large parts of the connective tissue. This fact is of particular importance during embryonic development, when solid structures have not yet developed. Another well-known example is the nucleus pulposus , the gelatinous nucleus of the intervertebral discs , which can therefore support large parts of the body's weight.
lubricant
Hyaluronic acid is the main component of synovia (joint fluid) and acts as a lubricant in all joint movements. It is also characterized by its structural viscosity properties: its viscosity changes with the acting mechanical forces; more precisely, the viscosity decreases the stronger the shear forces become. It is also liquid, but due to its high molecular weight it is viscous enough that it is not pressed out of the joint like water. In addition, due to chemical interactions and the external shape, it “adheres” particularly well to the cartilage of the joint.
If, at the beginning of a movement, for example in the knee joint when jumping or standing, strong compressive forces act on a joint, the molecules tangle together to form balls and hang on the surface of the cartilage like in a ball bearing. But if a quick shear movement is necessary, for example when running, the toughness of hyaluronic acid is reduced because of its structural viscosity and the friction is reduced.
Keeping paths clear
Hyaluronic acid keeps the “traffic routes” free for migrating cells. The migration of the cells is supported by widening the intercellular spaces (distances between the cells) .
Biochemical function
While the functions mentioned above relate to freely available hyaluronic acid, it is also involved in the formation of other, even larger, giant molecules, the proteoglycans . In particular, it links certain proteoglycans ( aggrecan in hyaline cartilage ) to form huge proteoglycan aggregates.
Function in the brain
In addition to important structural functions in the brain, it was shown that hyaluronan can influence the reconstruction of myelin sheaths around axons ( remyelination ). Another inhibitory function seems to play a role , especially in multiple sclerosis .
Interaction with receptors
A number of cell surface receptors interact with hyaluronic acid and trigger certain reactions in the cell, most notably cell division and migration. In the embryo , these stimulations are necessary when in contact with tumor cells , they can, however, also according to the organism adverse effects have.
Anti-carcinogenic effects in naked mole rats
Naked mole rats can live up to 30 years and develop practically no tumors. Long-chain hyaluronic acid is recognized as the cause. It is believed that the animals form these to care for their skin. It is assumed that the cancer-preventing property is a side effect. The normal, shorter version of hyaluronic acid is used on humans and is well tolerated. The long-chain one has not been tried.
Chemical structure
The hyaluronic acid is a macromolecular chain of disaccharides , which in turn consist of two glucose derivatives: D - glucuronic acid and N -acetyl- D -glucosamine . Both differ from β- D -glucose only in a substitution on the sixth or second carbon atom. In the disaccharide, the glucuronic acid is linked glycosidically β (1 → 3) to the N-acetyl- D -glucosamine, which in turn is linked glycosidically β (1 → 4) to the next glucuronic acid in the polymeric chain . A chain typically consists of 250 to 50,000 disaccharide units.
In neutral aqueous solution, hydrogen bonds are mainly formed between the carboxyl and the N -acetyl groups.
biosynthesis
In contrast to all other glycosaminoglycans, hyaluronic acid is not composed in the endoplasmic reticulum or Golgi apparatus , but rather from integral membrane proteins . Vertebrates have three types of these HA synthases , HAS1, HAS2 and HAS3. In the cell, these enzymes transfer the growing chain to ever new monosaccharide building blocks, which thus become longer and longer and are transported out of the cell through the membrane by ABC transporters . This does not apply to all HA synthases.
Use in human medicine
The sodium salt of hyaluronic acid ( sodium hyaluronate or sodium hyaluronate ) is used medicinally . Hyaluronic acid from animal raw material (eg. As cockscomb ) or biotechnologically from streptococci obtained cultures. Stabilized hyaluronic acids represent a special modification - for example by means of the so-called NASHA technology (NASHA stands for "non-animal stabilized hyaluronic acid") - which can be changed between less than one percent and up to approx. 20 to 30 percent depending on the manufacturer. The percentage of stabilization does not play a major role in the shelf life of the products; the type of stabilization is important. In the case of hyaluronic acid products obtained from rooster combs, allergic reactions can occur if there is an allergy to bird proteins.
Hyaluronic acid preparations are in osteoarthritis damaged injected joints to lubricate the joint and to act as a "shock absorber" (so-called viscosupplementation ). The half-life of hyaluronic acid products depends on the molar mass and is between 17 and 60 hours. Currently available hyaluronic acids differ in the number of injections required - 1, 3 or 5 injections depending on the product. There is no direct correlation between the number of injections and effectiveness, so products with fewer injections can be more effective than products with more injections. They have a similar effect to the use of NSAIDs or cortisone injections, but the effectiveness was different in different studies and the benefit-harm balance was often negative. A therapeutic value in osteoarthritis has not been proven according to the status of the 2003/04 meta-analysis. This contradicts a study on the treatment of facet joint osteoarthritis with hyaluronic acid from the observation period October 2007 to July 2009. The statutory health insurance companies usually do not cover the treatment costs. After reviewing the available studies, the medical service of the umbrella association of health insurers ( IGeL-Monitor ) rates a hyaluronic acid injection in knee osteoarthritis as "generally negative". Compared to placebo and no injections, it has been shown that pain can be reduced somewhat and the function of the joint slightly improved. However, mild adverse events at the injection site are common after hyaluronic acid injection. Serious side effects also occur - their frequency is unclear due to poor reporting in the studies.
The use of hyaluronic acid in hydrogels for wound care and in the treatment of chronic lung diseases is being investigated.
Hyaluronic acid is also used in combination with chondroitin sulfate and Poloxamer 407 in the treatment of heartburn , taking advantage of the fact that at body temperature this changes from a liquid to a semi-solid hydrogel and forms a physical barrier that attaches to the esophageal lining and prevents damage protects with stomach acid and pepsin . Hyaluronic acid and chondroitin sulfate provide rapid relief from reflux symptoms and also contribute to the regeneration and wound healing of the damaged mucous membrane.
Some nasal sprays contain hyaluronic acid to prevent the nasal mucous membranes from drying out. Hyaluronic acid is used in throat tablets to protect the lining of the mouth and throat. Hyaluronic acid is also used in eye drops for the treatment of " dry eyes ". The visco-elastic property of hyaluronic acid ensures a stable and long-lasting tear film without impairing vision. Therefore, hyaluronic acid is also used in cleaning and care solutions for contact lenses. It should keep the eye from drying out, even when wearing dimensionally stable and soft lenses for a long time, which should increase wearing comfort. In ophthalmic surgery , viscoelastic sodium hyaluronate solutions are used to fill the vitreous humor as well as to stabilize the anterior chamber and to protect the highly sensitive endothelial cell layer of the cornea during an operation on the anterior segments of the eye, especially cataract (cataract) surgery .
For some years now, products have been on the market that enable patients with stress urinary incontinence to be treated with stabilized hyaluronic acid. Here four hyaluronic acid depots are injected around the urethra. The procedure leads to an improvement in about half of the patients within the first year, but the long-term success of this treatment is low and the complication rate is high.
For children with vesicorenal reflux (VUR), preparations based on stabilized hyaluronic acid have been on the market for a long time, which are a good alternative to long-term drug therapy. The results here are promising.
Hyaluronan is also offered as a dietary supplement . In the food sector, v. a. enzymatic hydrolysates from chicken breast legs or rooster combs are used. These differ in the amount and type of co-formulants - some of which are declared as desired -: other glycosaminoglycans such as chondroitin sulfate or proteins and in the molar mass : e.g. B. "HCK" 250 kDa, Injuv (from rooster combs ) 50-200 kDa, Hyal-Joint (also from rooster combs) 6,000-9,000 kDa.
Most of the hyaluronic acid-containing supplements offered in Germany are marketed for joint function; they are multipreparations with other nutrients that are often better documented scientifically. Small amounts of 50 to 250 mg are administered daily via the preparations. An in vivo resorption study after oral administration with a molar mass of 1000 kDa (i.e. a relatively high molar mass) showed a certain oral availability. The hyaluronic acid that occurs in the plasma after oral administration has a lower molar mass (for example from about 30 to> 80 kDa).
In biochemistry, hyaluronic acid is used as a basis for growth in tissue engineering .
Use in cosmetics
Hyaluronic acid is used in cosmetics . However, since the hyaluronic acid in its full size is only slightly absorbed by the skin, degradation fragments of hyaluronic acid can be used. These components, known as HAF , have a lower molecular weight (e.g. 50 or 130 kilodaltons ), which means that the individual fragments can more easily penetrate the skin.
Trade names
Curavisc (D), Durolane (CH), RenehaVis (D), Fermavisc (CH), Hyalart (D), Hyalubrix (D), Hyalur (CH), HYGAG (D), Ial (CH), Ialugen (CH), Lacri-Vision (CH), Lacrycon (CH), Laservis (CH), Ostenil (D, CH), Recosyn (D), Rhinogen (CH), Sinovial (CH), Suplasyn (D, CH), Synvisc (D, CH), Unike-Injekt (D), Viscontour (D), Viscoseal (CH), Hylo-Vision (D), Visiol (CH), Vislube (CH), Vismed (CH), Xidan (D), numerous generics ( D, CH)
Alphastria (CH), Ialugen Plus (CH, with silver sulfadiazine ), Hyalofemme (D)
literature
- Per Hedén, Gabriella Sellman, Mats von Wachenfeldt, Michael Olenius, Dan Fagrell: Body Shaping and Volume Restoration: The Role of Hyaluronic Acid. In: Aesthetic Plastic Surgery. 33, 2009, p. 274, doi : 10.1007 / s00266-008-9303-y . PMID 19280248 . PMC 2693799 (free full text).
Web links
- Entry on hyaluronic acid at Vetpharm, accessed on November 23, 2011.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Robert Stern: Hyaluronan in cancer biology. 1st edition. Academic Press / Elsevier, San Diego 2009, ISBN 978-0-12-374178-3 , (online)
- ↑ D. Vigetti, E. Karousou, M. Viola, S. Deleonibus, G. De Luca, A. Passi: Hyaluronan: biosynthesis and signaling. In: Biochimica et Biophysica Acta . Volume 1840, number 8, August 2014, pp. 2452–2459, doi: 10.1016 / j.bbagen.2014.02.001 . PMID 24513306 .
- ↑ S. Back u. a .: Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation (abstract). In: Nature Medicine . 11, 2005, pp. 966-972.
- ↑ Marieke Degen : Age before beauty - hyaluronic acid protects naked mole rats from cancer. In: dradio research news . June 20, 2013.
- ↑ T. Schulz, U. Schumacher, P. Prehm: Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. In: J. Biol. Chem. 282, pp. 20999-21004.
- ↑ C. Hubbard, JT McNamara, et al. a .: The hyaluronan synthase catalyzes the synthesis and membrane translocation of hyaluronan. In: Journal of molecular biology . Volume 418, number 1–2, April 2012, pp. 21–31, doi: 10.1016 / j.jmb.2012.01.053 . PMID 22343360 .
- ↑ FOCUS Online: Hyaluronic acid is said to fill worn joints . In: FOCUS Online . ( focus.de [accessed on May 13, 2017]).
- ↑ HYALURONIC ACID (SUPLASYN UA): WHAT IS EVIDENCE? - arznei telegram. Retrieved May 13, 2017 .
- ↑ D. Trigkilidas, A. Anand: The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. In: Annals of the Royal College of Surgeons of England. Volume 95, Number 8, November 2013, pp. 545-551, doi: 10.1308 / 003588413X13629960049432 . PMID 24165334 .
- ↑ EC Rodriguez-Merchan: Intra-articular Injections of Hyaluronic Acid and Other Drugs in the Knee Joint. In: HSS journal: the musculoskeletal journal of Hospital for Special Surgery. Volume 9, Number 2, July 2013, pp. 180-182, doi: 10.1007 / s11420-012-9320-x . PMID 24426865 . PMC 3757486 (free full text).
- ↑ Arznei-Telegram 2002, No. 4 (PDF; 27 kB).
- ^ R. Raman, A. Dutta, N. Day, HK Sharma, CJ Shaw, GV Johnson: Efficacy of Hylan GF 20 and Sodium Hyaluronate in the treatment of osteoarthritis of the knee - a prospective randomized clinical trial. In: Knee. 15 (4), Aug 2008, pp. 318-324. Epub 2008 Apr 21.
- ↑ N. Bellamy, J. Campbell, V. Robinson, T. Gee, R. Bourne, G. Wells: Viscosupplementation for the treatment of osteoarthritis of the knee. In: Cochrane Database of Systematic Reviews. Issue 2, 2006, Art. No.:CD005321. doi: 10.1002 / 14651858.CD005321.pub2 PMID 16625635
- ↑ Arznei-Telegramm 2002, No. 4, conclusion (PDF; 27 kB).
- ↑ Arznei-Telegram 1/2004
- ↑ Study on the treatment of facet joint arthrosis with hyaluronic acid
- ↑ Surgeon magazine for resident surgeons: official body of the professional association of resident surgeons (BNC), issue 57, issue 3.2012, pp. 46–47.
- ↑ IGeL-Monitor: Hyaluronic acid injection for knee osteoarthritis. Created on: May 14, 2014, accessed October 31, 2018.
- ^ RD Price, MG Berry, HA Navsaria: Hyaluronic acid: the scientific and clinical evidence. In: Journal of plastic, reconstructive & aesthetic surgery: JPRAS. Volume 60, number 10, 2007, pp. 1110-1119, doi: 10.1016 / j.bjps.2007.03.005 . PMID 17466613 .
- ^ L. Allegra, S. Della Patrona, G. Petrigni: Hyaluronic acid: perspectives in lung diseases. In: Handbook of experimental pharmacology. Number 207, 2012, pp. 385-401, doi : 10.1007 / 978-3-642-23056-1_17 . PMID 22566234 .
- ↑ a b c V. Savarino, F. Pace, C. Scarpignato, the Esoxx Study Group: Randomized clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation . In: Alimentary Pharmacology & Therapeutics . tape 45 , no. 5 , March 2017, p. 631-642 , doi : 10.1111 / apt.13914 , PMID 28116754 , PMC 5347926 (free full text).
- ↑ Massimo P Di Simone, Fabio Baldi, Valentina Vasina, Fabrizio Scorrano, Maria Laura Bacci: Barrier effect of Esoxx® on esophageal mucosal damage: experimental study on ex-vivo swine model . In: Clinical and Experimental Gastroenterology . tape 5 , June 11, 2012, p. 103-107 , doi : 10.2147 / CEG.S31404 , PMID 22767997 , PMC 3387832 (free full text).
- ↑ a b B. Palmieri, A. Merighi, D. Corbascio, V. Rottigni, G. Fistetto: Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux . In: European Review for Medical and Pharmacological Sciences . tape 17 , no. 24 , December 2013, p. 3272-3278 , PMID 24379055 .
- ^ Cancer Therapy and Oncology International Journal (CTOIJ). Retrieved September 29, 2019 .
- ↑ Patrick du Souich, Antonio G. García, Josep Vergés, Eulàlia Montell: Immunomodulatory and anti-inflammatory effects of chondroitin sulphate . In: Journal of Cellular and Molecular Medicine . tape 13 , 8a, 2009, pp. 1451–1463 , doi : 10.1111 / j.1582-4934.2009.00826.x , PMID 19522843 , PMC 3828858 (free full text).
- ↑ M. Schnabelrauch, D. Scharnweber, J. Schiller: Sulfated Glycosaminoglycans As Promising Artificial Extracellular Matrix Components to Improve the Regeneration of Tissues. 2013, accessed on September 29, 2019 .
- ↑ Kessiena L. Aya, Robert Stern: Hyaluronan in wound healing: Rediscovering a major player . In: Wound Repair and Regeneration . tape 22 , no. 5 , 2014, p. 579-593 , doi : 10.1111 / wrr.12214 .
- ↑ F. Lone, AH Sultan, R. Thakar: Long-term outcome of transurethral injection of hyaluronic acid / dextranomer (NASHA / Dx gel) for the treatment of stress urinary incontinence (SUI). In: International urogynecology journal and pelvic floor dysfunction. Volume 21, Number 11, November 2010, pp. 1359-1364, doi: 10.1007 / s00192-010-1211-4 . PMID 20571764 .
- ↑ Lajos Balogh, Andras Polyak, Domokos Mathe, Reka Kiraly, Juliana Thuroczy, Marian Terez, Gyozo Janoki, Yaoting Ting, Luke R. Bucci, Alexander G. Schauss: Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in rats and dogs. In: J. Agric. Food Chem. 56 (22), 2008, pp. 10582-10593; doi: 10.1021 / jf8017029 .
- ^ Patent specification of Hyal Pharmaceutical Corp.
- ^ MN Collins, C. Birkinshaw: Hyaluronic acid based scaffolds for tissue engineering - a review. In: Carbohydrate Polymers . Volume 92, number 2, February 2013, pp. 1262-1279, doi: 10.1016 / j.carbpol.2012.10.028 . PMID 23399155 .
- ↑ A. Fakhari, C. Berkland: Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. In: Acta Biomaterialia. Volume 9, number 7, July 2013, pp. 7081-7092, doi: 10.1016 / j.actbio.2013.03.005 . PMID 23507088 . PMC 3669638 (free full text).
- ↑ T. Pavicic, GG Gauglitz, P. Lersch, K. Schwach-Abdellaoui, B. Malle, HC Korting, M. Farwick: Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. In: Journal of Drugs in Dermatology . Volume 10, Number 9, September 2011, pp. 990-1000. PMID 22052267 .
