Lysosome
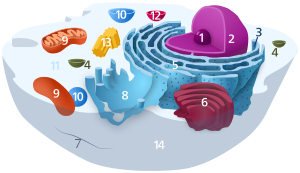
1. Nucleolus (nuclear body)
2. Cell nucleus (nucleus)
3. Ribosomes
4. Vesicle
5. Rough (granular) ER (ergastoplasm)
6. Golgi apparatus
7. Cytoskeleton
8. Smooth (agranular) ER
9 . mitochondria
10. lysosome
11. cytoplasm (with cytosol and cytoskeleton )
12. peroxisomes
13. centrioles
14 cell membrane
| Parent |
| Organelle |
| Subordinate |
| Lysosomes membrane lysosome Glycocalyx lumen protein complexes |
| Gene Ontology |
|---|
| QuickGO |
Lysosomes (from the Greek λύσις , from lysis 'solution', and σῶμα sṓma 'body') are cell organelles in eukaryotic cells . These are vesicles with an acidic pH value, enclosed by a simple biomembrane . They contain digestive enzymes and are partly produced in the Golgi apparatus . The function of the lysosomes is to break down biopolymers into their monomers .
construction
Lysosomes have a diameter of 0.1–1.1 μm. They contain many different hydrolyzing enzymes such as proteases , nucleases and lipases for the intracellular digestion of material . These enzymes are used for hydrolysis of proteins , polysaccharides , nucleic acids and lipids , so all major groups of macromolecules . These only achieve a high level of activity in an acidic environment with a pH of 4.5-5. This serves to protect the cell when a lysosome breaks open. In such a case, the enzymes would be inactive in the pH-neutral environment of the cytosol . This is an example of the importance of compartmentalization within the cell. The low pH value within the lysosomes is maintained by the lysosome membrane. Lysosomes are surrounded by a membrane with specific proteins. A V-type ATPase transports two protons (2H + ) into the lysosomes per ATP molecule . The membrane proteins are heavily glycosylated on the inside to protect against self-digestion.
Emergence
The hydrolytic enzymes and the lysosome membrane are formed by ribosomes on the rough (granular) endoplasmic reticulum (rER) and then transported to the Golgi apparatus . The lysosomal enzymes are sorted in the trans -Golgi apparatus and are packaged in a targeted manner in vesicles and transported to the later endosomes . In the case of hydrolases, a specific signal is known: mannose-6-phosphate groups (M6P) on exclusively nitrogen-coupled oligosaccharides . This modification takes place in the cis Golgi apparatus and is catalyzed by two enzymes: a phosphotransferase recognizes that it is a lysosomal enzyme and attaches N-acetylglucosamine -1-phosphate to one or two mannose residues ; the second enzyme cuts off the N-acetylglucosamine residue, with which the labeling is carried out.
In the trans -Golgi apparatus, the M6P residues are recognized by membrane-integrated M6P receptors . In the late endosome, the M6P receptors separate from their ligands again at pH 6 and are recycled.
There is also an M6P independent transport route into the lysosomes, e.g. B. in the membrane proteins of the lysosomes. The mechanism is not known.
tasks

Lysosomes digest non-cellular (heterophagy) but also cell-specific (autophagy) material. This also happens with programmed cell death .
Digestion of foreign material
Lysosomes are involved in digestion at the cellular level in several ways . By endocytosis resulting endosomes fuse with primary lysosomes to form secondary lysosomes. In protists this is called the food vacuole . In some cell types, fragments of the non-cellular material digested in the lysosome are presented on the cell surface in the form of so-called antigen fragments by MHC- II receptors. This process plays an important role in the immune system . Macrophages are an example of human cells that are capable of this .
Digestion of the cell's own material
The lysosomes not only utilize material that is foreign to the cell, but also its own material. This is called autophagy . Here, organelles or parts of the cytosol are broken down by the lysosome enzymes and reused. In this way, the cell renews itself with the help of the lysosomes. In a human liver cell, half of all macromolecules are broken down in this way every week.
Programmed cell death
The programmed cell death ( apoptosis ) by its own lysosome enzymes is an important task of the lysosomes. Apoptosis, for example, breaks down the tail of the tadpole in amphibians or the webbed feet between the fingers of the human embryo .
Diseases with lysosomal involvement
A defect in the phosphotransferase leads to a so-called lysosomal storage disease . Since it cannot be labeled with mannose-6-phosphate, the lysosomal enzymes are not sorted and reach the extracellular matrix in an uncontrolled manner via the plasma membrane ( I-cell disease , inherited as an autosomal recessive). Other lysosomal storage diseases are caused by defects in lysosomal hydrolases. This leads to an increase in non-degraded material in the lysosomes (e.g. Hunter's disease ). Mostly serious symptoms are the result. If LIPA mutates, Wolman's disease occurs . LIPA is the lysosomal, acidic lipase , which is important for the metabolism of cholesterol firsts and triglycerides .
Lysosomal accumulation of pharmaceuticals
Weak bases with lipophilic properties tend to accumulate in acidic intracellular compartments such as the lysosomes. While the plasma and lysosome membranes are permeable to the neutral, uncharged forms of such molecules, the charged, protonated forms of weak bases do not pass through the membranes and accumulate in lysosomes. Compared to the extracellular area, a 100 to 1000 times higher concentration of the weak bases can build up in the lysosome. This mechanism is called "lysosomotropy" or "acid trapping". The accumulation of lysosomotropic substances can be calculated using a cell-based mathematical model.
Many drugs used clinically are weak bases and accumulate in lysosomes. This can explain various pharmacological properties of such drugs. The tissue concentration of lysosomotropic drugs is well above the plasma concentration; and the half-life in tissue is higher than in plasma; Examples include haloperidol , levomepromazine or amantadine . In addition to the lysosomal accumulation, the lipophilicity of the substances and their absorption in fatty tissue structures also contribute to the high tissue concentrations and long tissue half-lives. Important lysosomal enzymes, such as acid sphingomyelinase , can be inhibited by drugs that have accumulated lysosomally. Such substances are called FIASMAs . This acronym is derived from the English term Functional Inhibitor of Acid SphingoMyelinAse ; this group of substances includes z. B. fluoxetine , sertraline or amitriptyline .
literature
- Bruce Alberts et al: Molecular Biology of the Cell. 4th edition. Garland Science, New York 2002, ISBN 0-8153-4072-9 .
- Neil A. Campbell, Jane B. Reece: Biology. 6th edition. Spectrum, Heidelberg 2003, ISBN 3-8274-1352-4 .
Web links
Individual evidence
- ^ WK Purves, et al .: Biologie , 7th edition, Spektrum Akademischer Verlag, 2006, ISBN 3-8274-1630-2 , p. 89.
- ↑ Online Mendelian Inheritance in Man database: Lysosomal acid lipase deficiency (# 278000). [1] .
- ↑ C. de Duve, T. de Barsy, B. Poole, A. Trouet, P. Tulkens, F. van Hoof. Lysosomotropic agents. In: Biochem. Pharmacol. 23: 2495-2531, 1974. PMID 4606365
- ↑ S. Trapp, G. Rosania, RW Horobin, J. Huber grain. Quantitative modeling of selective lysosomal targeting for drug design. In: Eur Biophys J . 37 (8): 1317-1328, 2008. PMID 18504571
- ↑ J. Kornhuber, A. Schultz, J. Wiltfang, I. Meineke, CH Gleiter, R. Zöchling, KW Boissl, F. Leblhuber, P. Riederer. Persistence of haloperidol in human brain tissue. In: Am J Psychiatry 156: 885-890, 1999. PMID 10360127
- ↑ J. Kornhuber, H. Weigmann, J. Röhrich, J. Wiltfang, S. Bleich, I. Meineke, R. Zöchling, S. Hartter, P. Riederer, C. Hiemke. Region specific distribution of levomepromazine in the human brain. In: J Neural Transm 113: 387-397, 2006. PMID 15997416
- ↑ J. Kornhuber, G. Quack, W. Danysz, K. Jellinger, W. Danielczyk, W. Gsell, P. Riederer. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. In: Neuropharmacology 34: 713-721, 1995. PMID 8532138
- ↑ J. Kornhuber, P. Tripal, M. Reichel, L. Terfloth, S. Bleich, J. Wiltfang, E. Gulbins. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. In: J Med Chem 51: 219-237, 2008. PMID 18027916
- ↑ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, TW Groemer, GM Spitzer, KR Liedl, E. Gulbins, P. Tripal. Identification of novel functional inhibitors of acid sphingomyelinase. In: PLoS ONE 6 (8): e23852, 2011. PMID 21909365
- ↑ J. Kornhuber, P. Tripal, M. Reichel, C. Mühle, C. Rhein, M. Muehlbacher, TW Groemer, E. Gulbins. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. In: Cell Physiol Biochem . 26: 9-20, 2010. PMID 20502000