Methane fermentation
As methane fermentation is referred to under anoxic conditions - ie in the absence of oxygen - running microbial degradation biotic , organic substances, in which, among other things , methane (CH 4 ) is the end product. It takes place in several phases of chemical transformations in which many different microorganisms of different metabolic types are involved and in which the formation of methane by archaea is the last step.
Occurrence
Methane fermentation occurs in oxygen-free (anoxic) areas where dead biomass is produced. Examples of this are swamps , rice fields overflowing with water , poorly aerated soils, and more or less deep layers of water sediments depending on the amount of biomass . Other examples are the digestive tracts of animals , especially ruminants and termites .
procedure
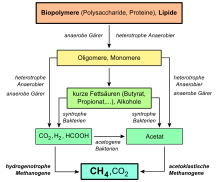
First and second breakdown phase: First, the various components of the biotic, organic substances are broken down by fermenting microorganisms to the typical end products of various fermentations . For this purpose, water-insoluble polymeric natural substances such as cellulose , proteins and nucleic acids must be split into water-soluble monomers by exoenzymes (enzymes excreted by the microorganisms into the medium) (first breakdown phase, hydrolysis phase). These are further broken down in different fermentations by different microorganisms ( bacteria and yeasts ) (second breakdown phase, fermentation phase). The end products of this first and second breakdown phase are alcohols (mainly ethanol , 2-propanol , butanol , 2,3-butanediol ), organic acids (mainly formic acid , acetic acid , propionic acid , butyric acid , lactic acid ), carbon dioxide (CO 2 ) and hydrogen ( H 2 ).
Third degradation phase (acetogenic phase): The fermentation end products of the second phase propionic acid, butyric acid, lactic acid and the alcohols are converted into acetic acid by acetogenic bacteria with elimination of hydrogen (H 2 ).
Fourth breakdown phase (methanogenic phase): In the last phase, the remaining breakdown products formic acid, acetic acid, carbon dioxide (partially) and hydrogen are converted by methanogenic archaea into methane and water (methane formation). Some of the carbon dioxide remains as an end product.
meaning
| source | Tg a −1 (month per year) | completely or mainly from methane fermentation |
|---|---|---|
| Swamps | 100-231 | Methane fermentation |
| ruminant | 76-189 | Methane fermentation |
| Rice crops | 31-112 | Methane fermentation |
| Natural gas and oil production, industry | 36-68 | |
| Biomass combustion | 14-88 | |
| Garbage, garbage | 35-69 | Methane fermentation |
| Coal mining | 30-48 | |
| Termites | 20-29 | Methane fermentation |
| Wildlife | 15th | Methane fermentation |
| Oceans | 4-15 | Methane fermentation |
| geological sources | 4-14 | |
| Methane hydrates | 4-5 | Methane fermentation |
| natural fires | 2-5 | |
| C3 plant cultures | 27 | |
| C4 plant cultures | 9 |
By far the largest part of the methane continuously formed on earth is formed microbially through methane fermentation (see table).
Methane is a climate-affecting gas. Although a large part of the methane formed ends up in oxic (oxygen-containing) water and soil areas and is oxidized there by methane-oxidizing bacteria with oxygen to form carbon dioxide and water, a considerable part of the methane reaches the higher atmosphere and has an impact on the climate there.
In the rumen of ruminants , methane fermentation produces large amounts of methane, which the ruminant body emits in gaseous form. The mass raising of cattle for the production of milk and beef causes the formation of significant amounts of methane in this way.
In technical plants, the methane-containing, gaseous end product biogas (around 60% methane, 35–40% carbon dioxide) is generated as an energy source by means of methane fermentation ( biogas plant , sewage treatment plant ).
Individual evidence
- ^ KL Denman, S. Brasseur, A. Chidthaisong, P. Ciais, PM Cox, RE Dickinson, D. Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S. Ramachandran, PL da Silva Dias , SC Wofsny, X. Zjang: Couplings between changes in the climate system and biogeochemistry. In: S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, KB Averyt, M. Tignor, HL Miller (eds.): Climate change 2007: The physical basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge UK, New York 2007, p. 542, table 7.6.
- ^ Matthias Koch: Ecological and economic evaluation of co-fermentation plants and their choice of location . KIT Scientific Publishing, 2009, ISBN 978-3-86644-355-6 ; P. 7.