Ninhydrin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
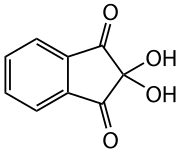
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ninhydrin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 6 O 4 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
Decomposition from 250 ° C |
|||||||||||||||
| solubility |
poor in water (20 g l −1 at 20 ° C)
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ninhydrin is the hydrate of indane-1,2,3-trione and is used as a reagent for the detection of ammonia and primary amino groups , especially amino acids .
properties
Ninhydrin reacts with the amino group of amino acids with elimination of water to form a Schiff base ( imine ). After decarboxylation of the carboxy group of the amino acid and subsequent cleavage of the amino residue, amino ninhydrin is formed. This dimerizes with ninhydrin to a violet dye (Ruhemann's purple). The intense purple color can be explained by mesomerism involving a hydrogen bond within the system of conjugated double bonds.
The intensity of the color is proportional to the concentration of the dye (see Lambert-Beer law ) and thus the concentration of an amino acid to be determined. For quantitative analysis , several samples with a known concentration are compared photometrically at 570 nm as standards with the sample to be examined. When reacting with secondary amino acids such as proline or hydroxyproline , a yellow color complex is formed, which can be measured photometrically at 400 nm.
Ninhydrin is also important in controlling the turnover in solid phase peptide synthesis (SPPS) and in organic solid phase synthesis (SPOS) and is part of the Kaiser test .
The reaction is relatively sensitive; the detection limit is 0.001 to 0.1 mg.
use
Ninhydrin is mainly used for the detection of amino acids and proteins . For proteins, however, the test is only successful if relatively short oligopeptides are present, since ninhydrin only reacts with free amino groups and these are hardly present in long-chain polypeptides. The detection is carried out in solution (with heating in a water bath). Ninhydrin is often used as a spray reagent z. B. used in paper chromatography or thin layer chromatography .
Another application based on this reaction is the creation of fingerprints . Since amino acids occur in sweat , they can react with ninhydrin and make finger and hand prints visible.
This mechanism is also used in medicine as the so-called Moberg test to detect peripheral nerve lesions. Since the sympathetic fibers that regulate sweat secretion run together with the peripheral nerve tracts from the exit from the spinal cord, sweat secretion is also disturbed, which can be demonstrated by a skin impression taken using ninhydrin.
An exception is the amino acid proline or hydroxyproline , which as secondary amino acids have a secondary (NH) amino group instead of a primary (NH 2 ) . Proline and hydroxyproline react via a different reaction mechanism to form a yellow-red product with an absorption maximum at 400 nm . When reacting with a primary amino acid, which has an NH 2 group, has an absorption maximum of 570 nm, and reacts to form a violet dye called Ruhemann's purple.
Reaction mechanism
⍺-amino acids
The reaction mechanism is more complicated than usually shown; Friedmann and Williams deal with this. In detail - as can be seen in the illustration - the following reactions take place: First the ninhydrin 1 is dehydrated to the trione. Amino group 2 of the amino acid attacks the middle carbonyl oxygen and condenses to form the imine . This has an open seven-membered ring structure, which enables a cyclic electron shift with decarboxylation . By hydrolysis of the imine, the amino acid residue is split off as an aldehyde and the primary amine remains. In a condensation reaction with a second ninhydrin molecule with elimination of water, the Ruhemanns purple 3 is created .
Exception: proline
A proline molecule and a ninhydrin molecule react to form a yellow addition product, which can now bind another ninhydrin molecule.
Ninhydrin in forensic use
Ninhydrin and Amido Black are used to secure fingerprints. A related patent was applied for on September 27, 1954 in the United States by Svante Odén , (born April 29, 1924 in Stockholm , † 1986 declaration of death after missing, professor of soil science). This gained fame through the discovery of the acidification of soils through precipitation and through analyzes of heavy metals in sewage sludge from municipal sewage treatment plants. Since then, the ninhydrin examination of absorbent trace carriers, in addition to the use of soot powder and cyanoacrylate, has been one of the three essential methods in forensic laboratories and is generally used as follows:
- Ninhydrin: 2 g of ninhydrin are dissolved in 100 ml of ethanol . The solution is acidified with approx. 0.5 g of 20% acetic acid and then sprayed onto the fingerprint with a sprayer. To develop the impression, it is held over steam for a few minutes. Ninhydrin reacts with the amino acids and proteins in sweat and forms a purple compound with a complex structure.
- NFN (Non Flammable Ninhydrin): The Non Flammable Ninhydrin solution causes a chemical reaction with some substances on the dactyloscopic trace. It works well with writing paper, newspapers, and cardboard; it is unsuitable for art print, glossy and photo paper. The trace carrier is immersed in the solution (the duration depends on the material thickness) or moistened with a cotton ball. After air drying, it is stored in the dark. The development of the traces takes 30 minutes to 72 hours; in urgent cases it can be accelerated by applying heat. Trace carriers treated with ninhydrin must immediately be placed in an airtight plastic cover, as touching them again would lead to further traces; In addition, harmful vapors or dusts can develop. Evidence is secured through photography.
- NPB (ninhydrin petroleum benzine): The solution causes a chemical reaction with some components of the dactyloscopic trace. The harmful components of NFN ( trichlorotrifluoroethane and acetic acid ) have been replaced by petroleum benzine , but the resulting vapor mixture is explosive. Electrical systems and devices in the work area must be explosion-proof. NPB works well with writing paper, newspapers, and cardboard; however, it cannot be used with art paper, glossy paper and photo paper. The processing takes place in the same way as with NFN
- Tetra-NPB (Tetra Ninhydrin Petroleum Benzine): The solution causes a chemical reaction with some components of the dactyloscopic trace. It is well suited for latent traces created by blood or blood water (the blood trace examination is not affected by the procedure). The mixture is poured into a jar until two layers have formed. The upper, light-colored liquid is poured off (must be disposed of), the lower, brown liquid is poured into the sprayer. The securing of evidence takes place in the original (with trace carrier) or photographically.
- Ninhydrin - Acetone: The solution causes a chemical reaction with some components of the dactyloscopic trace. It works well for writing paper, newspapers and cardboard; it cannot be used for art print, glossy or photo paper. Acetone dissolves almost all font coloring agents, stamps, etc. The method may therefore only be used if the examination of these trace types can be dispensed with. The search for clues is carried out in the same way as with NFN
- Sirchie - Onprint: The onprint spray is an industrial product that contains substances similar to NFN. It works well on writing paper, newspapers, and cardboard; it cannot be used on art paper, glossy paper and photo paper. The trace carrier is sprayed from a distance of 30 cm and air dried. When stored in the dark, the traces develop independently in a time of approx. 30 minutes to 72 hours.
particularities
Ninhydrin is one of the few compounds that contradict the Erlenmeyer rule.
literature
- Siegfried Ruhemann: Cyclic di- and tri-ketones. In: Journal of the Chemical Society Transactions . 97, 1910, pp. 1438-1449. doi: 10.1039 / CT9109701438
Web links
- Ruhemanns Purpur ( Memento from May 31, 2008 in the Internet Archive )
- Detection of amino acids in milk
- Reaction mechanism
Individual evidence
- ↑ a b entry on ninhydrin. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ a b c d Entry on ninhydrin in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ^ S. Ebel, HJ Roth (Ed.): Lexicon of Pharmacy. Georg Thieme Verlag, 1987, ISBN 3-13-672201-9 , p. 465.
- ^ E. Kaiser, RL Colescott, CD Bossinger, PI Cook: Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. In: Analytical Biochemistry . Volume 34, No. 2, April 1970, pp. 595-598.
- ↑ VK Sarin, SBH Kent, JP Tam, RB Merrifield: Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. In: Analytical Biochemistry. Volume 117, No. 1, p. 147.
- ↑ a b FU Berlin: Proteins. FU Berlin, accessed on November 11, 2019 .
- ↑ Mendel Friedman, L. David Williams: Stoichiometry of formation of Ruhemann's purple in the ninhydrin reaction . In: Bioorganic Chemistry . tape 3 , no. 3 , 1974, p. 267-280 , doi : 10.1016 / 0045-2068 (74) 90017-0 .
- ↑ Robert Heindl: Patent for a criminalistic process (ninhydrin method) . In: Archives for Criminology . tape 119 , 5 and 6. Schmidt-Römhild , Lübeck June 1, 1957, pp. 177 (text of the patent claim in Identification News , January 1957, pp. 1 f.).
- ↑ Understanding Chemistry: Fingerprints , accessed December 31, 2009.
- ^ A b c d Henry C. Lee, Robert E. Gaensslen: Advances in fingerprint technology. CRC Press, 2001.
- ↑ G. Kaiser: Kriminologie. An introduction to the basics. 10th, completely revised edition. Heidelberg 1997.



