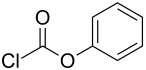
Phenyl chloroformate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phenyl chloroformate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 5 ClO 2 | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 156.57 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.24 g cm −3 |
|||||||||||||||
| Melting point |
-28 ° C |
|||||||||||||||
| boiling point |
188-189 ° C |
|||||||||||||||
| Vapor pressure |
0.9 h Pa (20 ° C) |
|||||||||||||||
| solubility |
reacts with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phenyl chloroformate is a chemical compound that serves as an intermediate in the production of various carbonic acid derivatives.
Chemical properties
Phenyl chloroformate belongs to the group of chloroformic acid esters , as well as ethyl chloroformate , methyl chloroformate , isopropyl chloroformate , n- butyl chloroformate and benzyl chloroformate .
use
Phenyl chloroformate is used as an intermediate in the production of the pesticides amidosulfuron , buprofezin , flazasulfuron , imazosulfuron , nicosulfuron , prosulfuron , pyrazosulfuron , rimsulfuron and thidiazuron as well as for further syntheses.
nomenclature
The common name "phenyl chloroformate" is incorrect, as it is a derivative of carbonic acid , not a derivative of formic acid . It is the monochloride and at the same time the monophenyl ester of carbonic acid.
Individual evidence
- ↑ a b c d e f g Data sheet for phenyl chloroformate synthesis (PDF) from Merck , accessed on May 3, 2014.
- ↑ a b Entry on phenyl chloroformate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 1029 ( limited preview in Google Book search).

