Sulfochlorination
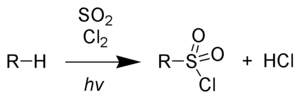
The sulfochlorination or Reed reaction is a chemical reaction triggered by light or radical initiators in which hydrogen in a hydrocarbon compound is replaced by the chlorosulfone group (-SO 2 Cl) with the formation of alkyl sulfonyl chlorides . The reaction is usually initiated photochemically and takes place in a radical chain reaction between alkanes, sulfur dioxide and chlorine .
These products of the reaction are processed into sulfonic acid amides or alkyl sulfonic acid salts , among other things , which are used as surfactants . Since a direct sulfonation of the alkanes with oleum or sulfur trioxide is hardly possible, this reaction has proven useful. Due to the chlorine bound directly to the sulfur , the resulting products are extremely reactive. The by-products in the reaction mixture are alkyl chlorides, which are formed by pure photochlorination , as well as multiple sulfochlorinated products. The reaction produces hydrogen chloride as a by- product .
history
The first description of sulfochlorination comes from Cortes F. Reed in 1936. The reaction is therefore also known as the Reed reaction. Reed assumed that the reaction proceeds via the formation of hypochlorosulfonyl chloride , an unstable isomer of sulfuryl chloride with tetravalent sulfur, in which chlorine is bound directly to the sulfur via a hypochlorite group. The hyposulfonium chloride should react with alkanes to form alkyl sulfite ester chlorides.
The actual radical chain mechanism was elucidated by Friedrich Asinger in the early 1940s . As head of the research group for the development of new surfactants in the central test laboratory of the Merseburg ammonia works in Leuna , he played a decisive role in the technical implementation of the process. Alkane fractions from the Fischer-Tropsch synthesis formed the raw material basis , which were converted into secondary alkyl sulfonates by sulfochlorination and then saponified. With these so-called mersolates , the supply of detergents was maintained in the Second World War without consuming valuable fats and oils .
background
A primary photochemical reaction is only triggered by absorbed light. Therefore one of the reactants has to absorb this light. In the case of sulfochlorination, the chlorine is the absorbing reactant. Chlorine absorbs light in a wavelength range of around 250 to 450 nanometers , corresponding to absorption in the long-wave ultraviolet and the visible violet spectral range . An energy of 244 kilojoules per mole is required for the homolytic splitting of chlorine .
According to the photochemical equivalence law , every absorbed photon causes a primary photochemical reaction.
with N A as Avogadro's constant (N A = 6.022 10 23 mol −1 ), Planck's quantum of action (h = 6.626 10 −34 Js) and the light frequency with the unit s −1 .
About the relationship:
with for the speed of light (c = 299,792,458 ms −1 ) results
and thus for the wavelength in the unit nm:
Inserting the values results in a value for the wavelength of 491 nanometers, which the incident light may have at most in order to cause chlorine to split. The absorption maximum of chlorine is around 340 nanometers. Light of this wavelength radiates an energy of about 377 kilojoules per mole and is therefore more than sufficient for photolysis of the chlorine. The amount of absorbed light is described by the Lambert-Beer law and depends on the concentration of the absorbing substance, the absorption coefficient of the material and the layer thickness.
For photochemical processes it is important how the number of converted molecules relates to the number of absorbed light quanta . This ratio is called the quantum yield (QA) at a certain wavelength of light. The ratio of converted molecules of the light-absorbing substance to the number of absorbed photons is calculated as:
The quantum yield should not exceed the value 1 in the photochemical primary process, the absorption of the light quantum. In the case of sulfochlorination, however, this ratio is not equal to or less than 1, but is often considerably higher because of the secondary processes in which the same substances are formed as in the primary photochemical process.
reaction
Alkanes react with a mixture of sulfur dioxide and chlorine to form alkyl sulfonyl chlorides:
Stoichiometric amounts of hydrogen chloride are formed as a by-product . The chain reaction is started photolytically by supplying suitable energy in the form of ultraviolet radiation . Higher-energy gamma radiation can also be used. This is how the homolytic break in the chlorine-chlorine bond occurs:
The chlorine molecule breaks down homolytically into two chlorine radicals . A chlorine radical reacts with the alkane to form an alkyl radical and hydrogen chloride. The alkyl radical then combines with the sulfur dioxide. Finally, the alkylsulfonyl chloride is formed with the release of a new chlorine radical. The chain reaction starts again and the chlorine radical formed in the last step reacts with the alkane to form an alkyl radical and hydrogen chloride. The chain is terminated by recombination of radicals. In addition to the alkanes, polymers such as polyethylene can also be sulfochlorinated.
Reaction engineering
The sulfochlorination takes place in the liquid phase by simultaneously introducing chlorine and sulfur dioxide into the liquid alkane, such as dodecane . The sulfochlorination is not selective ; a mixture of positional isomeric alkyl sulfonyl chlorides is often formed . The substitution of hydrogen atoms in a hydrocarbon is purely statistical, with tertiary hydrogen atoms reacting faster than secondary ones and these react faster than primary ones, with tertiary hydrogen preferably being subject to pure chlorination.
The only side reaction that occurs under certain conditions is photochlorination of the alkane:
This side reaction can be minimized by working with a slight, about ten percent excess of sulfur dioxide. The reaction is carried out at temperatures between 20 and 30 ° C, higher temperatures favor photochlorination.
In addition, multiple sulfochlorinated substances can form. If the reaction is carried out in daylight in the laboratory, chlorinated and sulfochlorinated products are formed in approximately the same ratio. By using ultraviolet light, the formation of by-products can be largely suppressed. Some of the products break down again into chloride and free sulfur dioxide under irradiation:
The reaction is terminated at a conversion of about 50% in order to minimize multiple substitution. The surfactants produced from multiple sulfochlorinated alkanes, especially those with an alkyl chain of up to 14 carbon atoms, have poor washing properties. In the case of longer-chain alkanes, the effect takes a back seat.
Products
Alkyl sulfonic acid salts
The alkylsulfonyl chlorides produced by this reaction are important intermediates in the industrial synthesis of detergents . The sodium salts of the alkylsulfonic acids are obtained by reaction with sodium hydroxide solution .
The chloroalkanes formed as a by-product are converted into water-soluble alcohols in this process step . The alkyl sulfonic acids are easily biodegradable under aerobic conditions . The alkyl sulfonic acids are used in emulsion polymerization and are also used to emulsify oils and mineral oils. The amine salts are oil-soluble and are used in the production of self-emulsifying oils such as the calcium or zinc salts of sulfonic acids.
Sulfonic acid amides
Sulfonic acid amides are formed through reaction with ammonia or alkylamines :
An excess of ammonia is necessary, as otherwise disulfimides will be formed:
Alkyl sulfonic acid esters
The alkyl sulfonyl chlorides can be converted to alkyl sulfonic acid esters with alcohols or sodium phenolate :
Sulfochlorinated polyethylene
Sulfochlorinated polyethylene can be crosslinked with benzidine to form rubber-like compounds. The brand name Hypalon from DuPont has become a common name for all chlorosulfonated polyethylenes (CSM for short), although there are other manufacturers of this product group.
Process variants
Working with radical starters
The sulfochlorination can also be carried out in a dark reaction in the presence of radical starters such as azobis (isobutyronitrile) . Peroxides such as hydrogen peroxide or acetone peroxide can also start the chain reaction.
Sulfobromination
When chlorine is replaced by bromine, there is no significant reaction to form sulfobromides. If you work with a mixture in which half of the bromine is replaced by chlorine on a molecular basis, alkanes react with carbon dioxide, bromine and chlorine to form sulfobromides, releasing hydrogen chloride.
Thiourea process
Alkylisothiourea hydrochlorides can be obtained by reacting chloroalkanes with thiourea with the release of hydrogen chloride. Thiourea reacts in the iso form. The alkyl isothiourea hydrochlorides react with chlorine in aqueous solution and convert to sulfochlorides.
literature
- E. Müller, O. Bayer, H. Meerwein, K. Ziegler: Houben-Weyl: Methods of Organic Chemistry. Vol. IX: Sulfur, Selenium, Tellurium Compounds , Thieme Verlag, 1955, ISBN 978-3-13-208104-8
Web links
Individual evidence
- ↑ Entry on sulfochlorination. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ Patent US2046090 : Method of halogenating compounds and product resulting therefrom. Published June 30, 1936 , inventor: Cortes F. Reed.
- ↑ Friedrich Asinger, Walter Schmidt, Franz Ebeneder: To the knowledge of the products of the joint action of sulfur dioxide and chlorine on aliphatic hydrocarbons in ultraviolet light, I. Mitteil .: The products of the joint action of sulfur dioxide and chlorine on propane in carbon tetrachloride solution. In: Reports of the German Chemical Society (A and B Series). 75, 1942, pp. 34-41, doi : 10.1002 / cber.19420750105 .
- ^ A b Egon Fanghänel: Prof. em. Dipl.-Ing., Dr. techn., Dr. phil. habil., Dr. rer. tech. hc, Dr. rer. nat. hc Friedrich Asinger (1907–1999) . In: Meeting reports of the Leibniz Society of Sciences in Berlin , 92 (2007), pp. 189–192.
- ^ A b E. Müller, O. Bayer, H. Meerwein, K. Ziegler: Houben-Weyl: Methods of Organic Chemistry. Vol. XIV / I: Macromolecular Compounds , Thieme Verlag, 1961, ISBN 978-3-13-214904-5 , p. 196.
- ↑ Hans Von Halban: The light absorption of chlorine. In: Journal of Electrochemistry and Applied Physical Chemistry. 28, 1922, pp. 496-499, doi: 10.1002 / bbpc.19220282304 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 71.
- ^ Arthur John Allmand: Part I. Einstein's law of Photochemical equivalence. Introductory address to Part I. In: Trans. Faraday Soc. 21, 1926, p. 438, doi: 10.1039 / TF9262100438 .
- ^ Theodor Weyl (original), Josef Houben (Hrsg.), Eugen Müller (Hrsg.): Methods of organic chemistry. IV / 5a photochemistry . Thieme Verlag, Stuttgart 1975, ISBN 978-3-13-201904-1 , p. 91.
- ↑ August Beer: Determination of the absorption of red light in colored liquids. In: Ann. Phys. Chem , 86.2, 1852, p. 78.
- ^ Johann Heinrich Lambert: Photometria, sive de mensura et gradibus luminis, colorum et umbrae . Sumptibus Vidae Eberhardi Klett, Augsburg 1760 ( digitized version available from the University of Strasbourg [accessed on May 16, 2016]).
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1364.
- ^ Alfred Schneider, Ju Chin Chu: Sulfochlorination of Cyclohexane Induced by Gamma Radiation. In: Industrial & Engineering Chemistry Process Design and Development. 3, 1964, p. 164, doi : 10.1021 / i260010a012 .
- ↑ MA Smook ET Pieski, CF Hammer: Derivatives of Chlorosulfonated polyethylenes and Their Infrared Spectra. In: Industrial & Engineering Chemistry. 45, 1953, p. 2731, doi : 10.1021 / ie50528a050 .
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009 , ISBN 978-0-471-70450-8 , p. 2311.
- ↑ Hans Kroepelin, AW Frh. Eberstein, W. Freiss, G. Käbisch, W. Opitz: Observations in the photochemical sulfochlorination of hydrocarbons. In: Angewandte Chemie. 64, 1952, pp. 273-274, doi : 10.1002 / anie.19520640908 .
- ↑ Friedrich Asinger, Franz Ebeneder, Gerd Richter: On the knowledge of the products of the joint action of sulfur dioxide and chlorine on aliphatic hydrocarbons in ultraviolet light. VI. About the influence of disulfonates on the surface-active and washing properties of monosulfonates. In: Journal for Practical Chemistry. 2, 1955, pp. 203-227, doi : 10.1002 / prac.19550020403 .
- ^ John Texter: Reactions and Synthesis in Surfactant Systems. , Marcel Dekker Inc., 2001, ISBN 978-0824702557 , p. 6.
- ^ E. Müller, O. Bayer, H. Meerwein, K. Ziegler: Houben-Weyl: Methods of Organic Chemistry. Vol. I / 2 General Laboratory Practice 1 . Thieme Verlag, 1959, ISBN 978-3-13-197404-4 , pp. 118-120.
- ^ Hype about discontinuing Hypalon
- ↑ George F. Lisk: Sulphonation. In: Industrial & Engineering Chemistry. 40, 1948, pp. 1671-1683, doi : 10.1021 / ie50465a019 .
- ^ Henry L. Wheeler, H. Stanley Bristol: Research in Pyrimidines: The Structure of Some Substitution Products . In: Amer. Chem. Jour. , 33, 437. Centr. B. 1905, I, 1709.
- ^ Treat B. Johnson, James M. Sprague: A New Method for the Preparation of Alkyl Sulfonyl Chlorides. In: Journal of the American Chemical Society. 58, 1936, p. 1348, doi : 10.1021 / ja01299a011 .