Rhodoces
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Rhodoces | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 10 Rh | |||||||||
| Brief description |
yellow solid (dimer) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 233.09 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
174 ° C (dec.) |
|||||||||
| solubility |
Dimer conditionally soluble in dichloromethane , soluble in acetonitrile |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
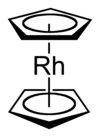
Rhodocene [Rh (C 5 H 5 ) 2 ], more precisely referred to as bis (η 5 -cyclopentadienyl) rhodium (II), is an organometallic compound from the series of metallocenes . In the molecule, a rhodium atom lies between two cyclopentadienyl rings in a sandwich complex .
properties
Rhodocene [Rh (C 5 H 5 ) 2 ] is a radical 19-electron complex that can only be found at temperatures above 150 ° C or by cooling to the temperature of liquid nitrogen (−196 ° C) . At room temperature (25 ° C) rhodocene in acetonitrile converts in less than 2 seconds by dimerization (combination) to [Rh (C 5 H 5 ) 2 ] 2 , a diamagnetic 18-valence electron complex in which two rhodocene units are connected to one another via cyclopentadienyl rings. Dimer rhodocene Rh (C 5 H 5 ) 2 ] 2 is a yellow solid.
Measurements by means of electron spin resonance spectroscopy (ESR), nuclear magnetic resonance spectroscopy (NMR) and infrared spectroscopy (IR) indicate that there is an equilibrium between the monomeric and dimeric forms. ESR data show that the monomer has a higher order axis symmetry C n , ( n > 2) with a mirror plane (σ) perpendicular to the plane of molecular symmetry, which means that the monomer has the typical sandwich structure of the metallocenes. The interpretation of these data has been questioned. The fragmentation route of the monomer was investigated by mass spectrometry . The dimerization is formally a redox reaction , the dimer is a rhodium (I) compound, while the monomer is a rhodium (II) compound. In stable complexes, rhodium usually occurs in the oxidation states + I or + III. The dimerization reduces the number of valence electrons on the rhodium nucleus from 19 to 18, since the oxidative coupling of the two cyclopentadienyl rings creates ligands with less hapticity . While in the monomer both Cp rings η 5 are bound to the central atom, in the dimer one ring is η 5 - and the other η 4 - bound. The η 4 -bound ring for the rhodium (I) metal center is a 4-electron donor, in contrast to the η 5 -bound 6-electron ligand. The increased stability of the dimeric 18-valence electron rhodium (I) complex compared to the monomeric 19-valence electron rhodium (II) complex also easily explains that the monomer can only be observed under extreme conditions. It was developed by El Murr et al. assumed and also inferred by Fischer and Wawersik on the basis of 1 H-NMR data that in the Rodocene dimer the hydrogen atoms of the connected Cp rings are in the endo position (i.e. facing towards the central atom). In contrast, Collins et al. assume that the hydrogen atoms are in the exo position.
Cotton and Wilkinson were able to show that the rhodocenium cation [Rh (η 5 -C 5 H 5 ) 2 ] + with 18 valence electrons can be reduced to the monomeric form in aqueous solution. However, they were unable to isolate the neutral product, not only because it dimerizes rapidly, but also because the rhodocene radical spontaneously converts to [(η 5 -C 5 H 5 ) Rh (η 4 -C 5 H 6 )] converts, a stable 18-electron rhodium (I) complex with mixed hapticity. This complex differs from the Rhodocene in two ways:
- a cyclopentadienyl ligand was created by adding a hydrogen atom to cyclopentadiene, which remains bound as an η 4 4-electron donor
- the rhodium (II) center is reduced to rhodium (I)
EO Fischer et al. assumed that this complex forms in separate steps through protonation and reduction. It could be shown that this complex can also be produced by reducing a Rhodocenium solution with sodium borohydride in aqueous ethanol . Here the product was characterized as biscyclopentadienylrhodium hydride.
Octaphenylrhodocene (a derivative with eight phenyl groups ) is the first substituted rhodocene to be isolated at room temperature, although this too decomposes rapidly in air. The X-ray structure analysis showed that octaphenylrhodocene has a sandwich structure with a staggered conformation . In contrast to cobaltocene , which has successfully found its way into research as a one-electron reducing agent , no rhodocene derivative has been discovered which has sufficient stability for such an application.
presentation
Only 2 years after ferrocene was discovered, the first Rhodocenium salts were made. In the first synthesis, cyclopentadienyl magnesium bromide (C 5 H 5 MgBr) was reacted with tris (acetylacetonato) rhodium (III) . Numerous other reaction pathways, including gas-phase redox transmetalation with ferrocene or nickelocene or the use of half-sandwich precursors have been reported since then.
Modern microwave synthesis methods have also been reported. Rhodocenium hexafluorophosphate is formed after reaction of cyclopentadiene with rhodium (III) chloride hydrate in methanol , followed by working up with methanolic ammonium hexafluorophosphate ; After only 30 seconds of microwave irradiation, the product is obtained with a yield of over 60%.
Due to their stability and the relatively simple preparation, rhodocenium salts are usually the starting substances for the synthesis of rhodocene and substituted rhodocene, all of which are unstable in themselves. Rhodocene can be z. B. by reacting a rhodocenium salt with molten sodium . The rhodecene can then be obtained as a black polycrystalline substance by sublimation on a finger cooled with liquid nitrogen . Upon warming to room temperature, it turns into a yellow solid, which can be identified as the rhodocene dimer.
use
Biochemists have studied the use of rhodium compounds and their derivatives in medicine and report the potential use of rhodocene derivatives as a radiopharmaceutical for the treatment of smaller areas of cancer . Rhodocene derivatives are also used for the synthesis of linked metallocenes, in which metal – metal interactions can be investigated. Possible applications of rhodocene derivatives are also molecular electronics and research into the mechanisms of catalysts .
Remarks
- ↑ The presence of a mirror plane perpendicular to the symmetry axes indicates an ecliptic instead of a staggered conformation. Free rotation of the cyclopentadienyl ligands along the metal Cp axis is common - in ferrocene the rotation barrier is ~ 5 kJ mol −1 . Therefore, in solution, staggered and ecliptic molecules should exist side by side and also merge quickly into one another. Therefore it is only really useful to specify a staggered or ecliptic conformation in the solid state.
- ↑ There are 2 different approaches to electron counting , depending on whether you are looking at radical or ionic species. Assuming radicals, the rhodium center has 9 electrons and every cyclopentadienyl ligand is a 5-electron donor. In the ionic approach, the cyclopentadienyl ligand is a 6-electron donor and the number of electrons in the rhodium center depends on the oxidation level: rhodium (I) has eight, rhodium (II) seven and rhodium (III) 6. Both approaches deliver the same result, but must be considered separately.
Individual evidence
- ↑ a b c d e f N. El Murr, JE Sheats, WE Geiger, JDL Holloway: Electrochemical Reduction Pathways of the Rhodocenium Ion. Dimerization and Reduction of Rhodocene . In: Inorg. Chem. . 18, No. 6, 1979, pp. 1443-1446. doi : 10.1021 / ic50196a007 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d e f g E. O. Fischer , H. Wawersik: About aromatic complexes of metals. LXXXVIII. About Monomeric and Dimeric Dicyclopentadienylrhodium and Dicyclopentadienyliridium and About a New Process for the Preparation of Uncharged Metal-Aromatic Complexes . In: J. Organomet. Chem. . 5, No. 6, 1966, pp. 559-567. doi : 10.1016 / S0022-328X (00) 85160-8 .
- ↑ a b c H. J. Keller, H. Wawersik: Spectroscopic investigations on complex compounds. VI. EPR spectra of (C 5 H 5 ) 2 Rh and (C 5 H 5 ) 2 Ir . In: J. Organomet. Chem. . 8, No. 1, 1967, pp. 185-188. doi : 10.1016 / S0022-328X (00) 84718-X .
- ↑ a b c B. De Bruin, DGH Hetterscheid, AJJ Koekkoek, H. Grützmacher: The Organometallic Chemistry of Rh-, Ir-, Pd-, and Pt-Based Radicals: Higher Valent Species . In: Prog. Inorg. Chem. . 55, 2007, pp. 247-354. doi : 10.1002 / 9780470144428.ch5 .
- ↑ DV Zagorevskii, JL Holmes: Observation of Rhodocenium and Substituted-Rhodocenium ion and Their neutral counterparts by Mass Spectrometry . In: Organometallics . 11, No. 10, 1992, pp. 3224-3227. doi : 10.1021 / om00046a018 .
- ^ Simon Cotton: Chemistry of Precious Metals. Springer Science & Business Media, 1997, ISBN 978-0-751-40413-5 , p. 78 ( limited preview in Google book search).
- ^ A b J. E. Collins, MP Castellani, AL Rheingold, EJ Miller, WE Geiger, AL Rieger, PH Rieger: Synthesis, Characterization, and Molecular-Structure of Bis (tetraphenylcyclopentadienyl) rhodium (II) . In: Organometallics . 14, No. 3, 1995, pp. 1232-1238. doi : 10.1021 / om00003a025 .
- ^ A b F.A. Cotton, RO Whipple, G. Wilkinson: Bis-Cyclopentadienyl Compounds of Rhodium (III) and Iridium (III) . In: J. Am. Chem. Soc. tape 75 , no. 14 , 1953, pp. 3586-3587 , doi : 10.1021 / ja01110a504 .
- ^ MLH Green, L. Pratt, G. Wilkinson: 760. A New Type of Transition Metal-Cyclopentadiene Compound . In: J. Chem. Soc. . 1959, pp. 3753-3767. doi : 10.1039 / JR9590003753 .
- ^ NG Connelly, WE Geiger: Chemical Redox Agents for Organometallic Chemistry . In: Chem Rev.. . 96, No. 2, 1996, pp. 877-910. doi : 10.1021 / cr940053x . PMID 11848774 .
- ^ DB Jacobson, GD Byrd, BS Freiser: Generation of Titanocene and Rhodocene Cations in the Gas Phase by a Novel Metal-Switching Reaction . In: J. Am. Chem. Soc. . 104, No. 8, 1982, pp. 2320-2321. doi : 10.1021 / ja00372a041 .
- ↑ HT He: Synthesis and Characterization of Metallocenes Containing Bulky Cyclopentadienyl Ligands 1999. , PhD thesis School of Chemistry, Faculty of Science, University of Sydney
- ↑ DR Baghurst, DMP Mingo: Design and Application of a reflux Modification for the Synthesis of Organometallic Compounds Using Microwave Dielectric Loss Heating Effects . In: J. Organomet. Chem. . 384, No. 3, 1990, pp. C57-C60. doi : 10.1016 / 0022-328X (90) 87135-Z .
- ↑ DR Baghurst, DMP Mingos, MJ Watson: Application of Microwave Dielectric Loss Heating Effects for the Rapid and Convenient Synthesis of Organometallic Compounds . In: J. Organomet. Chem. . 368, No. 3, 1989, pp. C43-C45. doi : 10.1016 / 0022-328X (89) 85418-X .
- ↑ Marcel Gielen: Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. Wiley, 2005, ISBN 978-0-470-86404-3 , doi : 10.1002 / 0470864052.ch20 , p. 379 ( limited preview in Google book search).
- ↑ M. Wenzel, YF Wu: Ferrocene, ruthenocene or. Rhodocene analogues of haloperidol synthesis and organ distribution after labeling with 103 Ru or. 103 m Rh . In: Int. J. Rad. Appl. Instrum. A. . 39, No. 12, 1988, pp. 1237-1241. doi : 10.1016 / 0883-2889 (88) 90106-2 . PMID 2851003 .
- ↑ M. Wenzel, YF Wu: Separation of [ 103 m Rh] rhodocene derivatives from the analogs [ 103 Ru] ruthenocene derivatives and their organ distribution . In: Int. J. Rad. Appl. Instrum. A. . 38, No. 1, 1987, pp. 67-69. doi : 10.1016 / 0883-2889 (87) 90240-1 . PMID 3030970 .
- ^ S. Barlow, D. O'Hare: Metal-Metal Interactions in Linked Metallocenes . In: Chem Rev.. . 97, No. 3, 1997, pp. 637-670. doi : 10.1021 / cr960083v .
- ↑ M. Wagner: A New Dimension in Multinuclear Metallocene Complexes . In: Angew. Chem. Int. Ed. . 45, No. 36, 2006, pp. 5916-5918. doi : 10.1002 / anie.200601787 .



![{\ displaystyle {\ ce {Rh (Acac) 3 {} + 2 (C5H5) MgBr -> [] [] [Rh (C5H5) 2] + {} + Acac ^ {-} {} + 2Mg (Acac) Br \ \ \ Acac = acetylacetonate}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5d2f324c359a58f3bf673f52b0aa874c2ac050a4)
![{\ displaystyle {\ ce {Rh + {} + M (C5H5) 2 -> [] [] [Rh (C5H5) 2] + {} + M \ \ \ \ \ M = Fe, Ni}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5983cbcc02f86a56899dbc61cc33bb52e6543b43)
![{\ displaystyle {\ ce {RhCl3.nH2O + 2C5H6 + NH4PF6 -> [Rh (C5H5) 2] PF6 + 2HCl + NH4Cl + nH2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1017a6637fda6885b7c83db24d558730206f1d18)