RuBisCO
| RuBisCO | ||
|---|---|---|

|
||
| RuBisCO molecule PDB 1RCX and 9RUB | ||
| Mass / length primary structure | 477 AS ; 52.7 kDa | |
| Secondary to quaternary structure | Hetero-16-mer (8 large + 8 small TU) | |
| Cofactor | Mg 2+ | |
| Identifier | ||
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 4.1.1.39 , lyase | |
| Substrate | D -ribulose-1,5-bisphosphate + CO 2 + H 2 O | |
| Products | 2 3- phospho - D- glycerate + 2 H + | |
Ribulose 1,5-bisphosphate carboxylase / -oxygenase , also known by the acronym-like shortcut RuBisCO is a for -dioxide fixation for example in plants important enzyme . It is responsible for the fact that all photosynthetically active plants and bacteria can absorb carbon dioxide , which is why it is probably the most abundant water-soluble protein on earth.
As a preliminary step in the Calvin cycle , RuBisCO adds a molecule of carbon dioxide (CO 2 ) to ribulose-1,5-bisphosphate . The resulting compound 2-carboxy-3-keto-D-arabinitol-1,5-bisphosphate breaks down with the addition of H 2 O into two phosphoglycerate molecules, which are further built up into carbohydrates. The energy for these reactions comes in the form of ATP from photosynthesis, i.e. from sunlight, or, as in the case of some chemolithotrophic bacteria, from the reactions of chemosynthesis ( chemotrophy ).
In addition to the CO 2 fixation , RuBisCO also catalyzes the incorporation of oxygen (O 2 ) as a side reaction . When the product is reused, energy and a carbon atom are lost as CO 2 , so this process is called photorespiration . In all organisms that carry out oxygenic photosynthesis, both reactions take place simultaneously, with the incorporation of CO 2 predominating. Some land plants are able to increase the efficiency of RuBisCO by separating metabolic pathways: C4 plants by spatial separation, CAM plants by temporal separation. Most algae and hornworts form pyrenoids , in which carbon dioxide is also accumulated locally. The activity of RuBisCO depends on the light. RuBisCO must be activated by a light-dependent activase before enzymatic activity.
It was discovered by Samuel Goodnow Wildman .
construction
RuBisCO consists of 16 subunits in plants, algae and cyanobacteria . There are eight so-called large sub-units (LSU or “L”, approx. 51,000–58,000 Da) and eight small sub-units (SSU or “S”, approx. 18,000 Da) as hexadecamer. In plants, RuBisCO is assembled in the chloroplasts of the cell. A special feature is that the eight identical large subunits in the chloroplast genome and the eight identical small subunits of the enzyme are encoded in the cell nucleus genome. In thale cress ( Arabidopsis thaliana ), the gene for the long chain is 1,440 base pairs long and, after transcription , translation and post-translational modification, results in the protein containing 477 amino acids . The short chains in Arabidopsis consist of 126 amino acids.
The quaternary structure of the most common form of the enzyme (type I, see section Orthologous forms ) is (L 2 ) 4 (S 4 ) 2 , the catalytic center being formed by a pair of the large subunits; each of these binds one of the product molecules 3-phosphoglycerate (3-PG; see Calvin cycle). The small subunits hold the complex together, but are unnecessary for the catalytic function. Presumably they also increase the specificity of the cylindrical holoenzyme.
Catalyzed reactions
Magnesium acts as a cofactor for RuBisCO . In the dark reaction ( Calvin cycle ) one molecule of ribulose-1,5-bisphosphate ( 1 ) is converted with carbon dioxide to two molecules of 3- D -phosphoglycerate ( 3 ). Ribulose-1,5-bisphosphate is in equilibrium with its enediol form ( 2 ) due to a keto-enol tautomerism , on which CO 2 condenses. After the reaction, the carbon atom of the carbon dioxide is now within the plant metabolism ( carbon dioxide fixation ).
Alternatively, RuBisCO also accepts oxygen. When oxygen is fixed, a molecule of 2-phosphoglycolate is produced instead ( 4 ), which is toxic in large quantities and must therefore be disposed of via photorespiration .
Land plants around the world fix an estimated 120 gigatons of carbon from CO 2 every year . This is around one sixth of the total atmospheric CO 2 and corresponds to around 17 to 20 times the amount of CO 2 released into the atmosphere annually by anthropogenic activities . Of this, around 1–2 gigatons of carbon are currently stored annually in the terrestrial ecosystems through the accumulation of biomass and organic matter in the soil. The rest is released back into the atmosphere through autotrophic and heterotrophic respiration. For fixation, 0.2% of the total protein occurring on earth is required (10 kg of RuBisCO are distributed evenly to each person on earth).
With a turnover rate of 17 / s (in the living cell: 3 / s) and the lossy side reaction of photorespiration, RuBisCO seems absurdly as one of the worst optimized (or mostly misunderstood) enzymes. Therefore, there has been no lack of attempts to change its properties using genetic engineering in order to achieve theoretical yield increases of up to 100%.
However, these experiments soon showed that any increase in the turnover rate was at the expense of specificity: the enzyme was less able to differentiate between oxygen and carbon dioxide, which promoted photorespiration. Conversely, an improved specificity led to a lower turnover rate and thus to a lower productivity. It appears that RuBisCO of a particular species is almost completely optimized for the prevailing environmental conditions (concentration of O 2 and CO 2 , temperature) despite its disadvantages mentioned above .
regulation
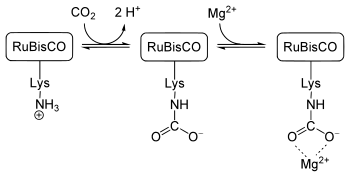
RuBisCO is regulated by a carbamatylation of an L - lysine . This lysine is located at position 201 of the large subunit. A carbon dioxide molecule reacts with the ε-amino group of the lysine to form a carbamate (see middle picture). RuBisCO is only active when the lysine is present as a carbamate and a magnesium ion binds to this carbamate (see picture on the right). This causes a change in conformation (the magnesium ion stabilizes this), as a result of which the large subunit can become enzymatically active.
The carbon dioxide molecule that reacts with the ε-amino group of the lysine to form a carbamate has nothing to do with the CO 2 molecule that is converted enzymatically in the carboxylase reaction (see above).
Orthologous forms
Four different forms of RuBisCO have been identified in nature that have different tertiary structures and kinetic characteristics:
- All green plants, algae and cyanobacteria have a Type I RuBisCO
- A form of RuBisCO type II has been discovered in some photosynthetically active proteobacteria, chemoautotrophic bacteria and dinoflagellates . Also Methanococcoides burtonii , an archaeon , has a RuBisCO of type II. It has no small subunits and forms a dimer. In addition, it has a higher turnover number than type I-RuBisCO, but is less specific to the incorporation of oxygen.
- The RuBisCO type III is found in archaea. Their tertiary structure largely corresponds to Type I or Type II, but special features have also been identified. The RuBisCO from Thermococcus kodakaraensis has a novel, ring-shaped structure made up of five large subunits. Type III enzymes are often adapted to high temperatures and have high turnover rates. For a long time it was a mystery why these archaea encode a type III RuBisCO enzyme but not ribulose-5-phosphate kinase. The latter catalyzes the formation of Ru-1,5-bP and is therefore a key enzyme in the Calvin cycle. However, it is postulated that archaea can synthesize Ru-1,5-bP in other ways, for example from 5-phosphoribosyl-1-pyrophosphate .
- There is also a so-called “RuBisCO-like enzyme”, which does not catalyze the fixation of carbon dioxide and is therefore not a bona fide RuBisCO. It is on methionine metabolism ( methionine salvage involved pathway). For example, the hyperthermophilic archaeon Archaeoglobus fulgidus has a “RuBisCO-like enzyme”.
literature
- Hans W. Heldt and Birgit Piechulla: Plant biochemistry . Spectrum Akademischer Verlag GmbH, 4th edition 2008; ISBN 978-3-8274-1961-3 ; P. 161ff.
- Caroline Bowsher, Martin Steer and Alyson Tobin: Plant Biochemistry . Garland Pub 2008; ISBN 978-0-8153-4121-5 ; P. 97ff.
- Tabita, FR. et al . (2008): Distinct form I, II, III, and IV RuBisCO proteins from the three kingdoms of life provide clues about RuBisCO evolution and structure / function relationships . In: J Exp Bot . 59 (7); 1515-1524; PMID 18281717 ; PDF (free full text access)
- Tabita FR. et al . (2008): Phylogenetic and evolutionary relationships of RuBisCO and the RuBisCO-like proteins and the functional lessons provided by various molecular forms . In: Philos Trans R Soc Lond B Biol Sci . 363 (1504); 2629-2740; PMID 18487131 ; PMC 2606765 (free full text)
Individual evidence
- ↑ UniProt entry
- ↑ GermOnline entry
- ↑ Tabita, FR, Hanson, TE, Li, H., Satagopan, S., Singh, J. and Tabita, SC (2007) Function, structure, and evolution of the RuBisCO-like proteins and their RuBisCO homologs . In: Microbiol Mol Biol Rev , 71 (4), 576-599; PMID 18063718 ; PMC 2168653 (free full text)
- ↑ Prentice, IC, GD Farquhar, MJR Fasham, ML Goulden, M. Heimann, VJ Jaramillo, HS Kheshgi, C. Le Quéré, RJ Scholes, DWR Wallace (2001). The Carbon Cycle and Atmospheric Carbon Dioxide. In: Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change [Houghton, JT, Y. Ding, DJ Griggs, M. Noguer, PJ van der Linden, X. Dai, K. Maskell, and CA Johnson (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 881pp.
- ↑ Turbo for biofuel , article in Chemie-Online from June 18, 2007
- ↑ Tcherkez, GG et al. (2006): Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized . In: Proc. Natl. Acad. Sci. Vol. 103, pp. 7246-7251. PMID 16641091 ; PDF (free full text access)
- ↑ Mueller-Cajar, O. and Badger, MR. (2007): New roads lead to RuBisCO in archaebacteria . In: Bioessays 29 (8); 722-724; PMID 17621634 ; doi : 10.1002 / bies.20616
- ↑ Sato, T. et al . (2007): Archaeal type III RuBisCOs function in a pathway for AMP metabolism . In: Science 315 (5814); 1003-1006; PMID 17303759 ; doi : 10.1126 / science.1135999
- ^ Finn, MW. and Tabita, FR. (2004): Modified pathway to synthesize ribulose 1,5-bisphosphate in methanogenic archaea . In: J Bacteriol. 186 (19); 6360-6366; PMID 15375115 ; PDF (free full text access)
- ↑ Ashida H. et al . (2003): A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO . In: Science 302 (5643); 286-290; PMID 14551435 ; doi : 10.1126 / science.1086997