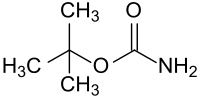
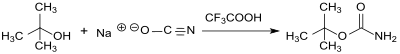tert -butyl carbamate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | tert -butyl carbamate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 11 NO 2 | |||||||||||||||
| Brief description |
beige to white crystalline solid or white needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 117.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.99 g cm −3 |
|||||||||||||||
| Melting point |
107-109 ° C |
|||||||||||||||
| boiling point |
196 ° C |
|||||||||||||||
| solubility |
soluble in dichloromethane , chloroform and alcohols, in DMF |
|||||||||||||||
| Refractive index |
1.428 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
tert -Butyl carbamate (Boc-amine) is the simplest Boc-protected amine and allows in the presence of palladium complexes - via the intermediate stage of the (hetero) aromatic tert -butyl carbamate - the direct introduction of an NH 2 group in halogen aromaticcompoundsor aryl triflate under gentle conditions in the sense of a Buchwald-Hartwig coupling .
Occurrence and representation
tert- Butyl carbamate is obtained in the reaction of tert-butanol and sodium cyanate in benzene or dichloromethane and in the presence of trifluoroacetic acid in a pure yield of 69%.
According to a worked out laboratory procedure, yields of 76 to 94% are achieved. The use of sodium cyanate is critical; Potassium cyanate only provides the product in a 5% yield.
The reaction of di-tert-butyl dicarbonate with ammonia in ethanol produces tert-butyl carbamate in 98% crude yield.
properties
tert -Butyl carbamate is a white, volatile, crystalline solid that dissolves in chlorinated hydrocarbons and alcohols .
Applications
Synthesis of nitrogen-containing heterocycles
The reaction of tert-butyl carbamate with cis -1,4-dichloro-2-butene in the presence of the base sodium hydride in DMF, in which the Boc-protected 3-pyrroline is formed in 60% yield, opens up a simple access to 3-pyrrolines .
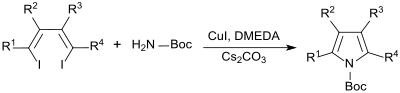
Highly substituted pyrroles can be prepared analogously by reacting 1,4-dihalo-1,3- dienes with tert-butyl carbamate in the presence of the catalyst copper (I) iodide , the copper ligand N, N'-dimethylethylenediamine (DMEDA) and the base cesium carbonate in Represent tetrahydrofuran as a solvent with very high yields.
Heteroarylpyrroles that are difficult to access on other synthesis routes, such as e.g. B. thienopyrroles can be obtained in this way in good yields.
The position of the halogen atoms on the double bond ( cis or trans configuration ) is unimportant for the reaction .
Multiply substituted pyrroles are also by a one-pot reaction of secondary propargyl alcohols with 1,3-dicarbonyl compounds, such as. B. ethyl acetoacetate and tert -butylcarbamate in the presence of ruthenium catalysts and trifluoroacetic acid TFA in useful yields (60 to 80%).
Also carbazoles may be prepared from the by Suzuki-Miyaura coupling readily available 2,2'-biphenyl ditriflate and tert -Butylcarbamat in the presence of palladium compounds and the phosphine ligand Xantphos be represented.
Substitution of halogen atoms on halogenated heteroaromatic compounds and halogenated aromatic compounds
On electron-rich heterocycles, such as. B. 3-bromofuran, the bromine atom can be replaced almost quantitatively by a Boc-protected amino group in a copper (I) iodide-catalyzed reaction in the presence of the DMEDA ligand by means of tert-butyl carbamate .
The substitution of halogen atoms on halogenated aromatics by a Boc-protected amino group by means of tert-butyl carbamate at room temperature - in the case of the aryl bromides - in the presence of palladium catalysts was first reported by John F. Hartwig in 1999. The aryl halide is reacted with tert-butyl carbamate in toluene , with sodium phenolate as the base, the ligand tri- tert -butylphosphine ( t -Bu 3 P) and the palladium catalyst bis (dibenzylideneacetone) palladium (0) (Pd (dba) 2 ).
Yields of 60 to 80% of carbamates are achieved.
This reaction, now known as the Buchwald-Hartwig coupling , was optimized by using the Pd (dba) 2 complex with chloroform as a catalyst, the phosphine ligand tert -butyl XPhos and the base sodium tert -butanolate to such an extent that up to good yields can be achieved.
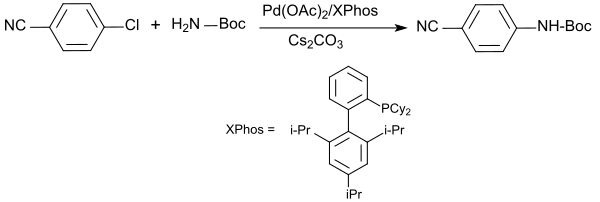
The amidation of halogenated aromatics and heteroaromatics with tert-butyl carbamate succeeds in the system cesium carbonate, 1,4-dioxane , palladium (II) acetate and the ligand XPhos ( dicyclohexyl (2 ', 4', 6'-triisopropylbiphenyl-2-yl) phosphine ) at 100 ° C in often excellent yields (> 90%).
The best results are achieved with bromoaryls, but chloroaryls also give usable yields with significantly longer reaction times.
Interestingly, this reaction can also be carried out in micellar solutions at room temperature (up to a maximum of 50 ° C.) with complete conversions and good pure yields.
With the nanomicell-forming surfactant TPGS-750-M, the Pd catalyst allyl palladium (II) chloride dimer and the base sodium tert -butylate in water at 40 ° C, z. B. in the reaction of 4-bromobenzophenone with tert-butyl carbamate, the corresponding Boc-protected amine is obtained in 98% yield (A), while the conventional reaction in the organic solvent toluene at 100 ° C gives a product yield of 90% (B) .
The aqueous medium can be recovered with high efficiency and used for renewed reactions.
Individual evidence
- ↑ a b c data sheet tert-Butyl carbamate from Sigma-Aldrich , accessed on September 10, 2017 ( PDF ).
- ↑ Entry on tert-butyl carbamate at TCI Europe, accessed on September 10, 2017.
- ↑ a b c B. Loev, MF Kormendy, MM Goodman: t-Butyl carbamate In: Organic Syntheses . 48, 1968, p. 32, doi : 10.15227 / orgsyn.048.0032 ; Coll. Vol. 5, 1973, p. 162 ( PDF ).
- ↑ a b c d e f A. Guingant, S. Collet: tert -Butyl Carbamate . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2010, doi : 10.1002 / 047084289X.m01188 .
- ↑ a b c Y. Tsuzuki, K. Chiba, K. Mizuno, K. Tomita, K. Suzuki: Practical synthesis of (3S, 4S) -3-methoxy-4-methylaminopyrrolidines . In: Tetrahedron Asymmetry . tape 12 , no. 21 , 2001, p. 2989-2997 , doi : 10.1016 / S0957-4166 (01) 00530-4 .
- ^ J. Yin, SL Buchwald: Palladium-catalyzed intermolecular coupling of aryl halides and amides . In: Org. Lett. tape 2 , no. 8 , 2000, pp. 1101-1104 , doi : 10.1021 / ol005654r .
- ↑ a b c J.F. Hartwig, M. Kawatsura, SI Hauck, KH Shaughnessy, LM Alcazar-Roman: Room-temperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic CN bond formation with a commercial ligand . In: J. Org. Chem. Band 64 , no. 15 , 1999, p. 5575-5580 , doi : 10.1021 / jo990408i .
- ↑ B. Loev, MF Kormendy: An improved synthesis of carbamates . In: J. Org. Chem. Band 28 , no. 12 , 1963, pp. 3421-3426 , doi : 10.1021 / jo01047a033 .
- ↑ a b R. Martin, CH Larsen, A. Cuenca, SL Buchwald: Cu-catalyzed tandem CN bond formation for the synthesis of pyrroles and heteroarylpyrroles . In: Org. Lett. tape 9 , no. 17 , 2007, p. 3379-3382 , doi : 10.1021 / ol7014225 .
- ↑ V. Cadierno, J. Gimeno, N. Nebra: One-pot three-component synthesis of tetrasubstituted NH pyrroles from secondary propargylic alcohols, 1,3-dicarbonyl compounds and tert -butyl carbamate . In: J. Heterocycl. Chem. Band 47 , 2010, p. 233-236 , doi : 10.1002 / jhet.301 .
- ↑ A. Kuwahara, K. Nakano, K. Nozaki: Double N-arylation of primary amine: Carbazole synthesis from 2,2'-biphenyldiols . In: J. Org. Chem. Band 70 , no. 2 , 2005, p. 413-419 , doi : 10.1021 / jo048472 + .
- ↑ MR Tracey et al .: Science of Synthesis: Houben-Weyl methods of molecular transformation, Vol. 21 . Georg Thieme, Stuttgart 2005, ISBN 3-13-118721-2 , p. 395 .
- ↑ S. Bhagwanth, AG Waterson, GM Adjabeng, KR Hornberger: Room-Temperature Pd-Catalyzed Amidation of Aryl Bromides Using tert -Butyl Carbamate . In: J. Org. Chem. Band 74 , no. 12 , 2009, p. 4634-4637 , doi : 10.1021 / jo9004537 .
- ↑ L. Qin, H. Cui, D. Zou, J. Li, Y. Wu, Z. Zhu, Y. Wu: Pd-catalyzed amidation of aryl (Het) halides with tert- butyl carbamate . In: Tetrahedron Lett. tape 51 , no. 33 , 2010, p. 4445-4448 , doi : 10.1016 / j.tetlet.2010.06.083 .
- ↑ NA Isley, S. Dobarco, BH Lipshutz: Installation of protected ammonia equivalents onto aromatic & heteroaromatic rings in water enabled by micellar catalysis . In: Green Chem. Band 16 , no. 3 , 2014, p. 1480-1488 , doi : 10.1039 / C3GC42188K .
- ↑ TPGS-750-M: Second-Generation Amphiphile for Organometallic Chemistry in Water at Room Temperature. In: sigmaaldrich.com. Sigma-Aldrich, accessed September 15, 2017 .