tert -dodecanethiol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
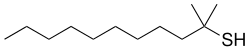
|
||||||||||||||||
| Structural formula of a structural isomer | ||||||||||||||||
| General | ||||||||||||||||
| Surname | tert -dodecanethiol | |||||||||||||||
| other names |
Dodecyl mercaptan |
|||||||||||||||
| Molecular formula | C 12 H 26 S | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 202.40 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.856 g cm −3 |
|||||||||||||||
| Melting point |
−45 ° C |
|||||||||||||||
| boiling point |
233 ° C |
|||||||||||||||
| Vapor pressure |
1.33 mbar (25.5 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (0.25 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
tert -Dodecanethiol is a chemical compound from the group of thiols . The technical product consists of a mixture of isomeric compounds in which the position of the thiol group on the alkyl radical consisting of 12 carbon atomsvaries.
Extraction and presentation
tert -Dodecanethiol can be produced by tetrapropylene with hydrogen sulfide .
composition
Tertiary dodecanethiol is, in the technical sense, a mixture of isomeric alkylthiols (common name: alkyl mercaptans) in which the mercaptan groups are each substituted by an alkyl radical with 12 carbon atoms, the carbon atoms carrying the mercaptan group being predominantly tertiary carbon atoms, i.e. in addition to the mercaptan group with three others Carbon atoms are connected. However, tert- dodecanethiol always also contains a certain proportion of secondary mercaptans in which the carbon atom carrying the mercaptan group is only bonded to two other carbon atoms and one hydrogen atom. Usually a smaller proportion, typically up to 20 percent by weight, of shorter or longer-chain alkyl radicals, for example alkyl radicals with 10, 11, 13 and / or 14 carbon atoms, is included. tert -Dodecanethiol consequently has the idealized semi- structural formula C 12 H 25 -SH, with a certain deviation from this idealized stoichiometric composition occurring depending on the content of alkyl radicals with more or fewer carbon atoms.
properties
tert -Dodecanethiol is a colorless, hardly inflammable liquid with an unpleasant odor, which is practically insoluble in water. It has a dynamic viscosity of 2.84 mPa · s at 20 ° C.
use
tert -Dodecanethiol is used as a synthetic chemical. It is mainly used as a molar mass regulator in polymerizations , in particular for free radical polymerizations of vinylic monomers such as butadiene , styrene , carboxylated styrene, acrylic acid , acrylonitrile , acrylic esters , vinyl ethers or their mixtures, especially in their emulsion polymerization in water.
safety instructions
The vapors of tert-dodecanethiol can form an explosive mixture with air ( flash point 98 ° C, ignition temperature 350 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on tert-dodecanethiol in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b Data sheet tert-dodecanethiol (mixture of isomers) (PDF) from Merck , accessed on September 18, 2011.
- ↑ Data sheet tert-dodecyl mercaptan, technical, ≥97.0% (RT) from Sigma-Aldrich , accessed on September 18, 2011 ( PDF ).
- ↑ a b Surechem: tert-dodecyl mercaptan ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
