Trametinib
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Trametinib | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class |
Kinase inhibitor |
||||||||||||
| Mechanism of action |
Reversible, allosteric inhibitor of MEK 1 and 2 in the MAP kinase signaling pathway |
||||||||||||
| properties | |||||||||||||
| Molar mass | 615.40 g · mol -1 | ||||||||||||
| Melting point |
300-301 ° C |
||||||||||||
| solubility |
practically insoluble in water in the pH range from 2–8 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Trametinib is a drug belonging to the group of kinase inhibitors. It is used alone or in combination with the drug dabrafenib to treat a certain type of skin cancer ( melanoma ) that has spread to other parts of the body or cannot be removed by surgery, and which causes a certain change ( mutation ) in position V600 of the so-called B-Raf gene. Trametinib is also used in combination with dabrafenib to treat advanced non-small cell lung cancer (NSCLC) with the B-Raf-V600 mutation.
Patents and drug approval
The first patent was granted in 2005 by Japan Tobacco Inc .; Tokyo, Japan filed with patent number WO 2005121142 (A1). In Germany, however, it is deposited under the number DE602005004286 (T2).
Trametinib was approved by the US Food and Drug Administration (FDA) in January 2014 under the trade name Mekinist ( GlaxoSmithKline ) for the treatment of non-resectable or metastatic melanoma with the B-Raf-V600E or V600K mutation in the US under the trade name Mekinist ( GlaxoSmithKline ) The approval was extended to the combination therapy with the B-Raf inhibitor dabrafenib. In the EU countries, Mekinist was approved in June 2014 and also includes the treatment of advanced non-small cell lung cancer.
In 2015, GlaxoSmithKline transferred approval to Novartis Europharm Ltd.
chemistry
properties
The drug trametinib has no stereocenters, but various low-energy conformations are favored. The general spatial configuration is illustrated by the following images.
It can be seen that the two aromatic rings are almost perpendicular to one another. The cyclopropane ring also protrudes from the plane of the basic structure, which consists of two linked, aliphatic rings that are slightly twisted against each other.
synthesis
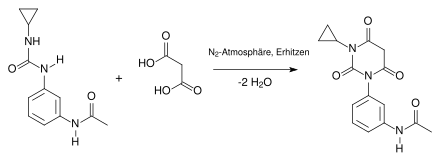
The synthesis takes place in several stages. In the first step, a urea derivative is formed from cyclopropylamine and the isocyanate derivative at room temperature in a nitrogen atmosphere . The nitrogen atom of the amine acts nucleophilically on the carbon atom of the isocyanate derivative:
The second step in the synthesis is a condensation reaction. You work in a nitrogen atmosphere and heat in the process. The urea derivative reacts with malonic acid , splitting off 2 molecules of water, to form a barbituric acid derivative :
In the third step of the synthesis, the oxygen atom of the barbituric acid derivative attacks the phosphorus atom of the phosphorus oxychloride nucleophilically . A mesomerism-stabilized complex is formed in which a double bond is formed, which is attacked by a chloride ion of the phosphorus oxychloride. Finally, dichlorophosphoric acid splits off and the halogenated product is obtained:
The fourth step of the synthesis is a nucleophilic substitution that proceeds according to the S N 1 mechanism. Hydrogen chloride (HCl) is formed as the elimination product:
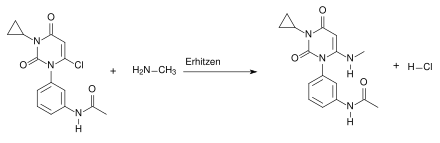
The fifth step of the synthesis is again a condensation reaction. It is heated again here. The product from step 4 reacts with the methylmalonic acid with elimination of water :
In the sixth step of the synthesis, methanesulfonyl chloride reacts with the hydroxyl group of the starting material and converts it into a good leaving group . The HCl formed is captured by the base triethylamine . Chloride then attacks the carbon by means of a nucleophilic substitution (S N 1). Methyl sulfonate is split off as a leaving group:
The seventh and final step of the synthesis is a nucleophilic substitution that proceeds according to the S N 1 mechanism. This produces HCl as the elimination product:
Analytics
Instrumental methods
Previously known, measured spectra (as of January 2014) are a 1 H- NMR spectrum and an MS - ESI spectrum.
Data of the 1 H-NMR spectrum ( DMSO-d6 , 400 MHz):
| Chemical shift δ ( ppm ) | |
|---|---|
| 1 | 0.63-0.70 (m, 2H) |
| 2 | 0.91-1.00 (m, 2H) |
| 3 | 1.25 (s, 3H) |
| 4th | 2.04 (s, 3H) |
| 5 | 2.58-2.66 (m, 1H) |
| 6th | 3.07 (s, 3H) |
| 7th | 6.92 (t, J = 8.8Hz, 1H) |
| 8th | 7.00–7.05 (m, 1H) |
| 9 | 7.36 (t, J = 8.2Hz, 1H) |
| 10 | 7.52–7.63 (m, 3H) |
| 11 | 7.79 (dd, J = 2.0, 10.4Hz, 1H) |
| 12 | 10.10 (s, 1H) |
| 13 | 11.08 (s, 1H) |
Data of the MS-ESI spectrum: MS / ESI m / e: 616 (M + H), 614 (MH)
Organic reactivity analysis
First, with the help of the Beilstein test, the iodine atom on the halogenated aromatic compound of the trametinib molecule can be detected as a general indication of halogens in organic compounds. The fluorine atom will probably remain unaffected due to the high binding force of the CF single bond, which is, however, irrelevant for this preliminary test.
After alkaline hydrolysis of the carboxamide function, acetate and a primary aromatic amine are formed as the functional group of the drug residue.
Detection options for the primary aromatic amine:
- Diazotization with nitrous acid (in situ with NaNO 2 and HCl) to the aromatic diazonium salt and subsequent coupling with Bratton-Marshall reagent (at weakly acidic pH) or β-naphthol (in weakly alkaline) to form the azo dye .
- Formation of colored imines or “Schiff bases” through reaction with Ehrlich's reagent (p- dimethylaminobenzaldehyde ).
- Folin reaction: Detection of a colored quinonimine , which is formed during the reaction of the primary aromatic amine with Folins reagent (1,2-naphthoquinone-4-sulfonate).
- Color reaction after arylation with Sanger reagent ( 1-fluoro-2,4-dinitrobenzene ). The fluorine atom of the reagent is nucleophilically attacked and substituted by the primary amino function.
- Organoleptic, due to the stinking smell of isonitrile, which is formed after the amine has reacted with chloroform in an alkaline environment (be careful, isonitriles are carcinogenic!)
After the hydrolysis of trametinib and subsequent preparation of the analytical batch, it is possible to distill off acetic acid and to detect this or its salts separately using the following identification reactions:
- Typical acetic acid odor; in the case of salts this is only noticeable through acidic vapors, which after protonation by z. B. oxalic acid and heating the approach.
- After esterification with ethanol at acidic pH, a characteristic odor also occurs, namely that of ethyl acetate.
- Formation of disgusting and poisonous cacodylo oxide, which is formed when salts of acetic acid react with arsenic.
- Formation of La (OH) · (CH 3 COO) 2 (= basic lanthanum acetate) after reaction of acetate with lanthanum nitrate. Ammonia is used as a solvent to set the necessary alkaline pH range of 9-11. Iodine atoms, which can be adsorbed on the lanthanum acetate formed, are likely to give the color in this reaction.
- Hydroxamic acid reaction: In the first step, acetic acid is converted into the acid chloride e.g. B. transferred with the help of thionyl chloride. This is followed by the reaction with hydroxylamine, which creates a hydroxamic acid derivative which forms three colored complexes with iron in the oxidation state.
The content can be determined either nitrometrically (diazo titration) or bromometrically (Koppe-Schaar titration) after the hydrolysis reaction. In the latter case, six equivalents of bromine are consumed.
Clinical information
application areas
Trametinib is indicated as monotherapy or in combination with dabrafenib for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation. Trametinib monotherapy has shown no clinical activity in patients whose disease progressed to prior therapy with a BRAF inhibitor.
Trametinib in combination with dabrafenib is also indicated for the treatment of adult patients with advanced non-small cell lung cancer with a BRAF V600 mutation.
Only patients in whom a BRAF V600E or V600K mutation had been detected were included in the pivotal studies. The names of the point mutations in the abnormal genes of those affected come from the fact that the amino acid valine (one-letter code: V) at position 600 of the B-Raf protein has been replaced by glutamic acid (E) or lysine (K). The anti-tumor or anti-proliferative effect is based on the inhibition of MEK kinases 1 and 2 in the MAP kinase pathway .
type of application
Mekinist is orally applied ( film-coated tablets ), it is dimethyl sulfoxide - solvate of trametinib (1: 1).
Therapy must be discontinued if the disease worsens or unacceptable adverse effects occur.
Contraindications and restrictions on use
Mekinist must not be administered if you are hypersensitive to trametinib . Renal disease is unlikely to have any clinical relevance to the pharmacokinetics of trametinib as the drug is mainly eliminated by the biliary route . For mild forms of pre-existing hepatic diseases, a dose adjustment is sufficient. However, studies are not yet available.
To date, no studies have been carried out with pregnant women or breastfeeding mothers. Since reproductive toxicity in the form of total loss of pregnancy or toxicity for the fetus has already been demonstrated in animal studies in lower systemic concentrations than are necessary for therapy, trametinib should not be taken during pregnancy. It is not known whether trametinib is excreted in breast milk. A risk for the child to be breast-fed cannot be ruled out, which is why it must be considered whether therapy with trametinib should be interrupted or breastfeeding stopped.
During and up to four months after treatment with a trametinib is contraception essential to the risk of pregnancy, the above mentioned reasons, be excluded.
Drug interactions
- Effects of trametinib on drug metabolizing enzymes and transporters
- In vitro studies show that trametinib is not an inhibitor of the following subfamilies of the cytochrome P450 enzyme system: CYP1A2, CYP2A6, CYP2B6, CYP2D6 and CYP3A4. The inhibition of CYP2C8, CYP2C9 and CYP2C19 only succeeded at concentrations that represent a multiple of the therapeutically required concentration (9 to> 100 times the amount), which is why no interactions with drugs that are metabolized via these enzymes are expected.
- It was also found that trametinib is an inhibitor of the OATP1B1, OATP1B3, BCRP transporters and P-glycoprotein in vitro. At the clinically relevant, systemic concentration of 0.04 μM, however, trametinib is not an inhibitor of the aforementioned transport proteins in vivo ,
- Effects of other drugs on trametinib
- Trametinib pharmacokinetics are not affected by other drugs. The metabolism by CYP enzymes is negligible and trametinib is not a substrate for the efflux transporters P-glycoprotein or BCRP. It is mainly deacetylated by hydrolytic enzymes, which are generally not associated with drug interactions.
- Drugs that increase the PR interval
- Mekinist can presumably extend the PR interval in a dose-dependent manner, which must be taken into account when taking drugs with the same effect. Are included antiarrythmics , β-blockers , calcium channel blockers (not of the dihydropyridine type), digitalis glycosides, sphingosine-1-phosphate receptor modulators and some HIV protease inhibitors .
unwanted effects
The following side effects have been found in clinical studies in the treatment of 329 patients with the above indication who took 2 mg Mekinist orally once daily . At least one of the side effects occurred in over 99% of all cases.
- Most common side effects (≥ 20%):
- Skin rash, acne-like skin inflammation, peripheral edema (lymphedema), diarrhea, tiredness, nausea, vomiting
- Common adverse effects (≥ 1%):
- Cellulitis, pulmonary embolism, anemia, shortness of breath, pneumonia, vomiting
- Side effects that led to permanent discontinuation of therapy (10%):
- Decrease in cardiac output , left ventricular dysfunction , pneumonia, increase in plasma levels of alanine aminotransferase
- Adverse effects that led to a dose reduction (26%) or short-term interruption of therapy (36%):
- Rash, acne-like skin inflammation, peripheral edema, drop in cardiac output, left ventricular dysfunction, diarrhea.
Pharmacological properties
Mechanism of action
Trametinib is a reversible, allosteric inhibitor of MEK1 and MEK2 ( mitogen-activated extracellular signal-regulated kinases 1 and 2 ) that inhibits both their activation and their kinase activity. The potency of the small molecule is illustrated by the IC 50 values of both the unphosphorylated forms of the serine / threonine kinases with 0.7 nM and 0.9 nM, and the information for the phosphorylated state with 13.2 nM and 10, 7 nM. MEK proteins (= MAPKK) are upstream regulators in the MAP kinase pathway . a. is involved in cell proliferation and differentiation as well as apoptosis . In the case of melanoma with the B-Raf-V600E or V600K mutation, this intracellular signaling pathway , which includes both the B-Raf protein (= MAPKKK) and MEK1 and 2, is permanently activated, leading to unbridled and uncontrolled cell growth Proliferation of cells leads. After administration of 1–2 mg trametinib, the inhibition of phosphorylated ERK (= MAPK) and Ki-67 ( biomarker for cell proliferation), as well as an increase in p27 (biomarker for apoptosis) was observed.
Furthermore, it was shown in vitro that trametinib, as a highly selective inhibitor of MEK1 and MEK2, has no affinity for other kinases at concentrations of up to 10 μM (6154 ng / ml). Furthermore, no significant binding activity (IC 50 > 10 μM, 6154 ng / ml) to numerous receptors, enzymes and ion channels could be determined during the screening .
Ingestion, distribution and elimination
Trametinib is rapidly absorbed after oral administration and reaches its maximum plasma concentration after 1.5 hours (t max ). A single dose of 2 mg of the drug has a bioavailability of 72% and a peak concentration (c max ) in plasma of 22.2 ng / ml. The volume of distribution is 214 liters. Trametinib is 97.4% bound to human plasma proteins .
Trametinib is mainly deacetylated in vitro , but is also mono-oxygenated and glucuronidated. Deacetylation is likely carried out by hydrolyzing carboxylesterases and amidases. The cytochrome P450 enzyme system is not involved in the metabolism. The starting substance is the compound that mainly circulates in the blood.
80% of the dose is excreted in the faeces, less than 20% in the urine. An amount of <0.1% of the eliminated dose represents the unmetabolized starting compound. The half-life is 5.3 days and the plasma clearance is 3.21 l / h.
Trade names
Monopreparations Mekinist (US)
Web links
- FDA: Approved Drugs: Trametinib ( Memento from June 19, 2013 in the Internet Archive )
- DailyMed : MEKINIST (trametinib) tablet, film coated
- MEKINIST (trametinib) tablets, for oral use
literature
- Keith T. Flaherty, Caroline Robert, Peter Hersey, Paul Nathan, Claus Garbe, Mohammed Milhem, Lev V. Demidov, Jessica C. Hassel, Piotr Rutkowski, Peter Mohr, Reinhard Dummer, Uwe Trefzer, James MG Larkin, Jochen Utikal, Brigitte Dreno, Marta Nyakas, Mark R. Middleton, Jürgen C. Becker, Michelle Casey, Laurie J. Sherman, Frank S. Wu, Daniele Ouellet, Anne-Marie Martin, Kiran Patel, Dirk Schadendorf: Improved Survival with MEK Inhibition in BRAF- Mutated melanoma . In: New England Journal of Medicine . tape 367 , no. 2 , July 12, 2012, p. 107-114 , doi : 10.1056 / NEJMoa1203421 .
- Jeffrey R Infante, Leslie A Fecher, Gerald S Falchook, Sujatha Nallapareddy, Michael S Gordon, Carlos Becerra, Douglas J DeMarini, Donna S Cox, Yanmei Xu, Shannon R Morris, Vijay GR Peddareddigari, Ngocdiep T Le, Lowell Hart, Johanna C Bendell, Gail Eckhardt, Razelle Kurzrock, Keith Flaherty, Howard A Burris, Wells A Messersmith: Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial . In: The Lancet Oncology . tape 13 , no. 8 , August 2012, p. 773-781 , doi : 10.1016 / S1470-2045 (12) 70270-X .
- Gerald S Falchook, Karl D Lewis, Jeffrey R Infante, Michael S Gordon, Nicholas J Vogelzang, Douglas J DeMarini, Peng Sun, Christopher Moy, Stephen A Szabo, Lori T Roadcap, Vijay GR Peddareddigari, Peter F Lebowitz, Ngocdiep T Le, Howard A Burris, Wells A Messersmith, Peter JO Dwyer, Kevin B Kim, Keith Flaherty, Johanna C Bendell, Rene Gonzalez, Razelle Kurzrock, Leslie A Fecher: Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose -escalation trial . In: The Lancet Oncology . tape 13 , no. 8 , August 2012, p. 782-789 , doi : 10.1016 / S1470-2045 (12) 70269-3 .
- J. Jing, J. Greshock, JD Holbrook, A. Gilmartin, X. Zhang, E. McNeil, T. Conway, C. Moy, S. Laquerre, K. Bachman, R. Wooster, Y. Degenhardt: Comprehensive Predictive Biomarker Analysis for MEK Inhibitor GSK1120212 . In: Molecular Cancer Therapeutics . tape 11 , no. 3 , March 6, 2012, p. 720-729 , doi : 10.1158 / 1535-7163.MCT-11-0505 .
- A. Vultur, J. Villanueva, C. Krepler, G. Rajan, Q. Chen, M. Xiao, L. Li, PA Gimotty, M. Wilson, J. Hayden, F. Keeney, KL Nathanson, M. Herlyn: MEK inhibition affects STAT3 signaling and invasion in human melanoma cell lines . In: Oncogene . April 29, 2013, doi : 10.1038 / onc.2013.131 .
Individual evidence
- ↑ Hiroyuki Abe, Shinichi Kikuchi, Kazuhide Hayakawa, Tetsuya Iida, Noboru Nagahashi, Katsuya Maeda, Johei Sakamoto, Noriaki Matsumoto, Tomoya Miura, Koji Matsumura, Noriyoshi Seki, Takashi Inaba, Hisashi Kawasaki, Reinay Kaki, Takayuki Yamomaguchuda, Japan Kurachi, Yoshikazu Hori, Takayuki Yoshida, Junya Kakegawa, Yoshihiro Watanabe, Aidan G. Gilmartin, Mark C. Richter, Katherine G. Moss, Sylvie G. Laquerre: Discovery of a Highly Potent and Selective MEK Inhibitor: GSK1120212 (JTP-74057 DMSO Solvates) . In: ACS Medicinal Chemistry Letters . 2, No. 4, April 14, 2011, pp. 320-324. doi : 10.1021 / ml200004g .
- ↑ MEKINIST (trametinib) tablets, for oral use
- ↑ There is not yet a harmonized classification for this substance . What is shown is a label for N- {3- [3-cyclopropyl-5 - [(2-fluoro-4-iodophenyl) amino] -6,8-dimethyl-2,4,7-trioxo- 3,4,6,7-tetrahydropyrido [4,3-d] pyrimidin-1 (2H) -yl] phenyl} acetamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 20, 2017.
- ↑ WO2005121142 (A1) - 2005-12-22 In: worldwide.espacenet.com .
- ↑ DE602005004286 (T2) - 2009-01-02 In: worldwide.espacenet.com .
- ↑ ( page no longer available , search in web archives )
- ↑ document WO2005121142 (A1) - 2005-12-22 In: worldwide.espacenet.com .
- ↑ Mekinist EU Product information. (PDF) European Medicines Agency, accessed on July 4, 2017 (English).
- ↑ AG Gilmartin, MR Bleam, A. Groy, KG Moss, EA Minthorn, SG Kulkarni, CM Rominger, S. Erskine, KE Fisher, J. Yang, F. Zappa Costa, R. Annan, D. Sutton, SG Laquerre: GSK1120212 (JTP-74057) Is an Inhibitor of MEK Activity and Activation with Favorable Pharmacokinetic Properties for Sustained In Vivo Pathway Inhibition . In: Clinical Cancer Research . tape 17 , no. 5 , March 1, 2011, p. 989-1000 , doi : 10.1158 / 1078-0432.CCR-10-2200 .
- ↑ April KS Salama, Kevin B Kim: Trametinib (GSK1120212) in the treatment of melanoma . In: Expert Opinion on Pharmacotherapy . 14, No. 5, April 2013, pp. 619-627. doi : 10.1517 / 14656566.2013.770475 .