Tris (hydroxymethyl) nitromethane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
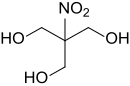
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tris (hydroxymethyl) nitromethane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 9 NO 5 | ||||||||||||||||||
| Brief description |
white to yellowish crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 151.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.5652 g cm −3 at 25 ° C |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| solubility |
very soluble in water (220 g · l −1 at 20 ° C), readily soluble in ethanol , very little soluble in benzene |
||||||||||||||||||
| Refractive index |
1.4183 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Tris (hydroxymethyl) nitromethane is the product of the complete conversion of nitromethane with formaldehyde and is structurally similar to the formaldehyde- releasing agent bronopol , whose bromine atom in THNM has been replaced by a hydroxymethyl group . The compound is used as a biocide for the preservation of liquid technical products and as a starting material for chemical syntheses.
Occurrence and representation
As early as 1895, the Belgian chemist Louis Henry described the nitroaldol reaction , later called the Henry reaction , of nitromethane with excess formaldehyde in the presence of a basic catalyst, e.g. B. Sodium Hydroxide .
The reaction is exothermic and, even if stoichiometric masses of formaldehyde are used, usually a mixture of mono-, di- and tri-addition products. If formaldehyde is used above stoichiometric, discoloration occurs due to (tar-like) polymers , and the excess formaldehyde adhering to the solid has to be laboriously removed during work-up.
For this reason, continuous processes have been proposed which essentially aim at reducing the residence time at elevated temperature in the reactor with slightly more than stoichiometric formaldehyde use. Yields of> 97% can be achieved in continuous operation under controlled conditions.
properties
As a pure product, tris (hydroxymethyl) nitromethane is a white, odorless crystal powder that dissolves very well in water and in polar alcohols, such as. B. Ethanol dissolves. From higher alcohols such as B. amyl alcohol , the crude product can be recrystallized. When dissolved in water (0.1 mol·l −1 ), a pH of 4.5 is established, at which trimethylolnitromethane is stable, i.e. H. is ineffective when used as a biocide. At pH values> 7.5, THNM slowly splits off formaldehyde, whereby the bactericidal effect begins.
Applications
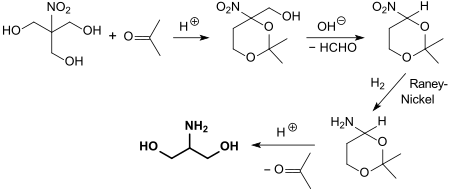
The functional amine 2-amino-1,3-propanediol (serinol), a structural element of the antibiotic chloramphenicol and precursor for sphingosines , fungicides and medicinal substances , is made from trimethylnitromethane after ketal formation with acetone , cleavage of the still free hydroxymethyl group , hydrogenation to the amino group and cleavage of the Ketal protecting group accessible.
The structural similarity of tris (hydroxymethyl) nitromethane to glycerine suggests its use as a starting substance for explosives. The resulting trinitrate ester is a secondary explosive .
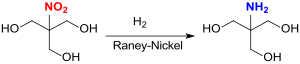
The buffer substance TRIS is obtained in the catalytic hydrogenation of tris (hydroxymethyl) nitromethane in methanol with Raney nickel in up to 90% yield.
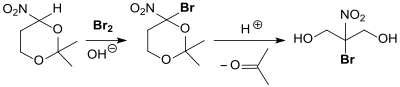
The synthesis route for the biocide bronopol also takes place via the serinol intermediate 2,2-dimethyl-5-nitro-1,3-dioxane, the nitro group of which is exchanged for a bromine atom . The protective group is then split off in acid. The total yield, starting from THNM, is approx. 73%.
The main application of tris (hydroxymethyl) nitromethane is as a formaldehyde releaser or formaldehyde donor for the biocidal equipment of aqueous systems such as e.g. B. adhesives and glues, cooling lubricants in metalworking, drilling muds and fracking fluids in the oil and gas industry, pot preservatives for latex paints , polymer emulsions , and as disinfectants , slime control agents and deodorants for circulating industrial water systems, e.g. B. in the pulp and paper industry. Typical concentrations in preparations are between 50 and 1,000 ppm. To increase the biocidal effect, mixtures with isothiazolinones or glutaraldehyde are often used. In addition, applications of THNM for viscosity control in cosmetic preparations, as crosslinkers in polyurethanes, are described.
safety instructions
Tris (hydroxymethyl) nitromethane is - in contrast to many other formaldehyde releasers - practically only added to technical products for preservation. The acute and subchronic oral and dermal toxicity values of tri (hydroxymethyl) nitromethane are toxicologically insignificant and Ames tests for mutagenicity were negative.
Isolated findings on sensitization to trimethylolnitromethane show a very low prevalence of 0 to 0.6% .
According to the Technical Rules for Hazardous Substances (TRGS) developed by the BAuA in the TRGS 611 edition, 2-hydroxymethyl-2-nitro-1,3-propanediol may be used as a "nitrosating agent" because of the risk of the formation of carcinogenic nitrosamines (since 1993) in Germany cannot be used in cooling lubricants.
Individual evidence
- ↑ a b c d e Entry on 2- (hydroxymethyl) -2-nitro-1,3-propanediol at TCI Europe, accessed on April 04, 2020.
- ↑ a b c Entry on tris (hydroxymethyl) nitromethane in the GESTIS substance database of the IFA , accessed on April 04, 2020 (JavaScript required)
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Amsterdam, NL 2015, ISBN 978-0-12-800834-8 , pp. 57 .
- ↑ a b Data sheet Tris (hydroxymethyl) nitromethane from Sigma-Aldrich , accessed on April 04, 2020 ( PDF ).
- ^ Louis Henry: Formation synthétique d'alcools nitrés . In: Comptes rendus . tape 120 , p. 1265-1268 ( bnf.fr ).
- ^ L. Henry: A propos des alcools nitrés . In: Recueil des Travaux Chimiques des Pays-Bas . tape 16 , no. 8 , 1897, p. 250-252 , doi : 10.1002 / recl.18970160803 .
- ↑ Patent DE1910458 : Process for the production of tris (hydroxymethyl) nitromethane. Registered on March 1, 1969 , published November 6, 1969 , applicant: Commercial Solvents Corp., inventor: HS Vierk, WA the nobility.
- ↑ Patent US2301259 : Method of producing nitrohydroxy compounds. Filed October 25, 1939 , published November 10, 1942 , applicant: Hercules Powder Co., inventor: RFB Cox.
- ↑ Patent EP0348223A2 : Novel process for the preparation of serinol. Filed June 23, 1989 , published December 27, 1989 , Applicant: WR Grace & Co.-Conn., Inventor: JM Quirk, SG Harsy, CL Hakansson.
- ↑ Patent US4233245 : Preparation of tris (hydroxymethyl) aminomethane. Applied on January 25, 1979 , published on November 11, 1980 , Applicant: Societé Chimique de La Grande Paroisse, Azote et Produits Chimiques, inventors: J. Bourguignon, M.-X. Sion, M. Moreau.
- ↑ Patent US4851588 : Novel process for the preparation of bronopol. Applied July 23, 1988 , published July 25, 1989 , Applicant: WR Grace & Co.-Conn., Inventors: RJ Kupper, F. Jachimowicz, JM Quirk, CL Hakansson.
- ↑ a b Tris (hydroxymethyl) nitromethane. In: Product Safety Assessment. The Dow Chemical Company, November 12, 2014, accessed April 6, 2020 .
- ↑ Patent EP2700313B1 : Biocidal compositions comprising glutaraldehyde and tris (hydroxymethyl) nitromethane and methods of use. Filed February 26, 2014 , published December 23, 2015 , applicant: Dow Global Technologies LLC, inventor: B. Yin.
- ↑ a b A. de Groot, J. Geier, M.-A. Flyvholm, G. Lensen, P.-J. Coenraads: Formaldehyde-releasers: relationship to formaldehyde contact allergy. Metalworking fluids and remainder. Part 1 . In: Contact Dermatitis . tape 63 , no. 3 , 2010, p. 117–128 , doi : 10.1111 / j.1600-0536.2010.01714.x .
- ↑ Tris Nitro®. In: Technical Bulletin. Angus Chemical Company, 1998, accessed April 7, 2020 .
- ↑ Restrictions on the use of water-miscible or water-mixed cooling lubricants, the use of which may result in N-nitrosamines. In: TRGS 611.BAUA , May 2007, accessed on April 7, 2020 .