Tropolones
The tropolones are a group of natural substances that are derived from 2-hydroxytropone (α-tropolone, also just tropolone for short ). The seven-membered carbocyclic ring system with three conjugated double bonds , one carbonyl and one hydroxyl group (6π- aromatics ) is characteristic.
Classification and occurrence
The basic structure of the seven-membered ring of α-tropolons is found in some natural products . The name “Tropolone” is derived from “ Atropine ” and was coined in 1945 by MJS Dewar for a group of oxyketones based on a novel ring system.
According to Nozoe, tropolones of the turpentine type, hydroxytropolonecarboxylic acids , purpurogalline and tropolones of the alkaloid type are distinguished.
- Tropolone terpene type : Among the simplest derivatives of tropolone counts the hinokitiol (C 10 H 12 O 2 ), and β-thujaplicin mentioned, the different in the wood cypress family occurs, so in the Taiwan Hinoki ( . Chamaecyparis obtusa var formosana ), from whose essential oil (hinoki oil ) it was isolated in 1935 by Tetsuo Nozoe in Taiwan . Hinokitiol is also found in the wood of the giant tree of life ( Thuja plicata ), in the Hiba tree ( Thujopsis dolabrata ), in cedar juniper ( Juniperus cedrus ) and in other types of cypress family. Hinokitiol also has an isopropyl group as a substituent on the ring system and thus has 10 carbon atoms like a monoterpene .
- Hydroxytropolone carboxylic acids : A different type of tropolone derivative is stipitats acid (C 8 H 6 O 5 ), with a carboxy and another hydroxyl group as substituents, which was isolated from the mold Penicillium stipitatum . The tropolones stipitatonic acid , puberulic acid and puberulonic acid are also produced by Penicillium species.
- In the case of purpurogallin (C 11 H 8 O 5 ), which occurs in various forms of galls such as gall apples and in the bark of oaks, the tropolone's structural motif is part of a bicyclic ring system.
- Tropolones of the alkaloid type : The ring system of colchicine , the main alkaloid of Colchicum autumnale ( autumn crocus ), can also be regarded as a complex tropolone derivative.
Parent compound α-tropolone
α-tropolone is the 2- hydroxy derivative of tropone and has two positional isomers with the same empirical formula (C 7 H 6 O 2 ). In the case of α-tropolone, the carbonyl and hydroxyl groups are adjacent on the ring in the 1,2-position. This enables a keto-enol tautomerism (see section properties), so that there are none of the usual ketone derivatives. In addition, β-tropolone (1,3-substitution) and γ-tropolone (1,4-substitution) are known, but they are of no particular importance.
| Tropolones | ||||||
| Surname | α-tropolone | β-tropolone | γ-tropolone | |||
| other names | 1,2-tropolone, 2-hydroxycyclohepta-2,4,6-trienone, purpurocatechol 2-hydroxytropone |
1,3-tropolone 3-hydroxytropone |
1,4-tropolone 4-hydroxytropone |
|||
| Structural formula |

|

|

|
|||
| CAS number | 533-75-5 | 3324-76-3 | 4636-39-9 | |||
| ? (Mixture of isomers) | ||||||
| PubChem | 10789 | 20751 | ||||
| Molecular formula | C 7 H 6 O 2 | |||||
| Molar mass | 122.12 g mol −1 | |||||
| Physical state | firmly | |||||
| Brief description | light yellow solid | |||||
| Melting point | 50-52 ° C | |||||
| boiling point | 80-84 ° C (0.1 mmHg) | |||||
| p K s value | 6.9 | 5.4 | ||||
| solubility | soluble in water | |||||
|
GHS labeling |
|
|
|
|||
| H and P phrases | no H-phrases | see above | see above | |||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||
| no P-phrases | see above | see above | ||||
| Toxicological data | 190 mg kg −1 ( LD 50 , rat , ip ) | |||||
synthesis
The oxidation of cycloheptanone with selenium dioxide leads to 1,2-cycloheptanedione. The subsequent bromination-dehydrobromination in a basic medium and a hydrogenation leads to α-tropolone.
properties
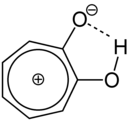
The α-tropolone occurs in two tautomeric forms, which has been proven by infrared spectra . Since the tautomeric conversion rate is very high, a structural formula with a hydrogen bond is also postulated. The change of the proton from one oxygen atom to the other leads to a shift of the π-electrons of the double bonds , which in a newly built seven-membered ring with 6π-electrons leads to the formation of a mesomerism-stabilized system which fulfills the Hückel rule , i.e. is an aromatic:
Reactivity
Like many other aromatics, α-tropolone can be nitrated and brominated . It couples with diazonium salts . It is isomerized to benzoic acid by heating .
Individual evidence
- ↑ a b c J. Falbe, M. Regitz (ed.): Römpp Lexikon Chemie, 10th edition, volume 6: T - Z . Thieme, Stuttgart 1999, p. 4687 ( limited preview in Google Book search).
- ↑ G. Huber: The tropolone and its derivatives . In: Angewandte Chemie , 1951, 63, pp. 501-508; doi: 10.1002 / anie.19510632102 .
- ^ Tetsuo Nozoe: Natural Tropolones and Some related Troponoids . In: Progress in the Chemistry of Organic Natural Products / Progress in the Chemistry of Organic Natural Products / Progrès dans la Chimie des Substances Organiques Naturelles. Ed .: L. Zechmeister. Springer Verlag, Vienna 1956 ( limited preview in the Google book search).
- ^ I. Murata, S. Itô, T. Asao: Tetsuo Nozoe: Chemistry and Life. In: Chemical Record. Volume 12, No. 6, December 2012, doi: 10.1002 / tcr.201200024 , PMID 23242794 .
- ↑ R. Hegnauer: Chemotaxonomy of Plants, Volume I. Springer Verlag, Basel 1962, p. 127 ( limited preview in Google Book search).
- ↑ Entry on colchicine. In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- ↑ a b c d e data sheet Tropolone at Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ a b J.S. Siegel, Y. Tobe (Ed.): Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 45a: Monocyclic Arenes, Quasiarenes, and Annulenes . Thieme, Stuttgart 2009, p. 364 ( limited preview in Google Book search).
- ^ Hans Beyer and Wolfgang Walter: Textbook of Organic Chemistry , S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , pp. 619–620.