1,4-pentanediol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,4-pentanediol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 O 2 | |||||||||||||||
| Brief description |
clear, colorless and oily liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.9883 g cm −3 |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
very easily soluble in water, methanol , ethanol and chloroform |
|||||||||||||||
| Refractive index |
1.4452 (23 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,4-Pentanediol is a chiral diol with a primary and a secondary hydroxyl group . It is accessible from the bio-based platform chemical levulinic acid by catalytic hydrogenation and could e.g. B. suitable as a diol component for polyester from renewable raw materials .
Occurrence and representation
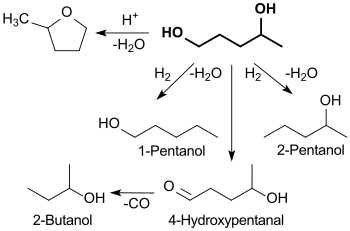
The formation of 1,4-pentanediol during the catalytic hydrogenation of levulinic acid on a copper - chromium oxide contact under the conditions of the then still common "fire and sword chemistry" (hydrogen pressures up to 267 atm ≈ 27 M Pa and temperatures up to 300 ° C) in 44% yield (in addition to 11% γ-valerolactone ) was reported in 1947. 1,4-pentanediol was also obtained from γ-valerolactone under similarly drastic conditions in yields of 32 to 83%.
The widely varying yields and low selectivities indicate a complex reaction process that proceeds via several intermediate products to 1,4-pentanediol - and further to the end product 2-methyltetrahydrofuran .
Depending on the hydrogenation conditions and catalysts, further (undesired) secondary products, such as 1-pentanol, 2-pentanol and 2-butanol, can be formed at the expense of the 1,4-pentanediol yield.
Intensive research in the recent past aimed to increase the selectivity of the hydrogenation of levulinic acid to 1,4-PDO under milder conditions, mostly using expensive precious metal catalysts such as rhodium or palladium or complex phosphine complex ligands .
Hydrogenation over a MoO x molybdenum oxide-modified ruthenium fixed bed catalyst on activated carbon in water at 70 ° C and 4 MPa hydrogen pressure, however, already gives promising values on a laboratory scale with 97% yield of 1,4-pentanediol with quantitative levulinic acid conversion.
An alternative approach to 1,4-pentanediol from renewable raw materials could be the catalytic hydrogenation of furfural , whereby also a ruthenium contact on mesoporous carbon at 80 ° C and 1 MPa H2-pressure 1,4-pentanol in 90% iger Yield is generated.
properties
1,4-Pentanediol is an oily, hygroscopic liquid that mixes very well with water and polar organic solvents.
Applications
1,4-Pentanediol has been described as a diol component in biodegradable polyorthoesters for the controlled release of active ingredients.
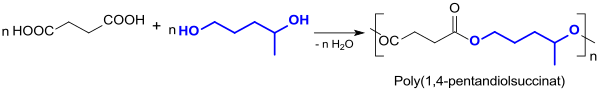
1,4-Dihydroxypentane has recently been proposed several times as a building block for polyester from renewable raw materials, but has not yet demonstrated its suitability convincingly. While the model polyester polybutylene succinate with a melting point of 115 ° C can be thermoplastically processed into molded parts and composite materials , the 1,4-PDO-based polyesters with bio-based dicarboxylic acids described so far occur as yellow, sticky oils or brittle substances.
The less reactive secondary hydroxyl group prevents the formation of higher molar masses and the pendant methyl group disrupts the crystallization of the molecular chains. The polyesters obtained thus were Differential scanning calorimetry (Engl. Differential scanning calorimetry , DSC) no melting points found. In polycarbonates and polyurethanes , too, there is currently no discernible application potential for 1,4-pentanediol.
Individual evidence
- ↑ a b c d Entry on 1,4-pentanediol at Toronto Research Chemicals , accessed December 30, 2018 ( PDF ).
- ↑ a b c d e f William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-438 .
- ↑ Data sheet 1,4-Pentanediol from Sigma-Aldrich , accessed on December 30, 2018 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ A. Phanopoulos, AJP White, NJ Long, PW Miller: Catalytic transformation of levulinic acid to 2-methyltetrahydrofuran using ruthenium N -triphos complexes . In: ACS Catal. tape 5 , no. 4 , 2015, p. 2500-2512 , doi : 10.1021 / cs502025t .
- ^ RV Christian, HD Brown, RM Hixon: Derivatives of γ-valerolactone, 1,4-pentanediol and 1,4-d- (β-cyanoethoxy) -pentane . In: J. Amer. Chem. Soc. tape 69 , no. 8 , 1947, pp. 1961–1963 , doi : 10.1021 / ja01200a036 .
- ↑ a b S.C. Patankar, GD Yadav: Cascade engineered synthesis of γ-valerolactone, 1,4-pentanediol, and 2-methyltetrahydrofuran from levulinic acid using Pd-Cu / ZrO 2 catalyst in water as solvent . In: ACS Sust. Chem. Eng. tape 3 , no. 11 , 2015, p. 2619-2630 , doi : 10.1021 / acssuschemeng.5b00763 .
- ^ A b J. Cui, J. Tan, Y. Zhu, F. Cheng: Aqueous hydrogenation of levulinic acid to 1,4-pentanediol over Mo-modified Ru / activated carbon catalyst . In: ChemSusChem . tape 11 , no. 8 , 2018, p. 1316-1320 , doi : 10.1002 / cssc.201800038 .
- ↑ M. Li, G. Li, N. Li, A. Wang, W. Dong, X. Wang, Y. Cong: Aqueous phase hydrogenation of levulinic acid to 1,4-pentanediol . In: Chem. Commun. tape 50 , 2014, p. 1414-1416 , doi : 10.1039 / c3cc48236g .
- ↑ A. Phanopoulos, AJP White, NJ Long, PW Miller: Catalytic transformation of levulinic acid to 2-methyltetrahydrofuran using ruthenium N -triphos complexes . In: ACS Catal. tape 5 , no. 4 , 2015, p. 2500-2521 , doi : 10.1021 / cs502025t .
- ^ F. Liu et al .: Catalytic cascade conversion of furfural to 1,4-pentanol in a single reactor . In: Green Chem. Band 20 , no. 8 , 2018, p. 1770-1776 , doi : 10.1039 / C8GC00039E .
- ↑ J. Heller et al .: Development of poly (ortho esters) and their application for bovine serum albumin and bupivacaine delivery . In: J. Control. Release . tape 78 , no. 1–3 , 2002, pp. 133-141 , doi : 10.1016 / S0168-3659 (01) 00482-5 .
- ^ F. van der Klis, RJI Knoop, JH Bitter, LAM van den Broek: The effect of Me-substitutents of 1,4-butanediol analogues on the thermal properties of biobased polyesters . In: J. Polym. Sci., Part A: Polym. Chem. Band 56 , no. 17 , 2018, p. 1903-1906 , doi : 10.1002 / pola.29074 .