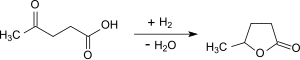
γ-valerolactone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
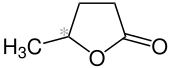
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | γ-valerolactone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 8 O 2 | |||||||||||||||
| Brief description |
clear, colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−31 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure | ||||||||||||||||
| solubility |
miscible with water, miscible with ethanol |
|||||||||||||||
| Refractive index |
1.4310-1.4340 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
γ-valerolactone ( gamma-valerolactone , GVL , 5-methyloxolan-2-one) is the furanoid lactone of 5-hydroxy-valeric acid (5-hydroxypentanoic acid). γ-valerolactone is as a platform chemical from renewable raw materials considered the future as monomer for polyesters , as a "green" solvents, as well as biogenic fuel and biogenic fuel could be used.
Manufacturing
The intramolecular cyclization of allylacetic acid (4-pentenoic acid) - accessible from allylacetoacetate via allylacetoacetic acid - with iron triflate generated in situ from iron (III) chloride and silver trifluoromethanesulfonate gives γ-valerolactone at 80 ° C in 1,2-dichloroethane in 93% iger yield.
1,4-pentanediol - e.g. B. by hydrogenation of α-angelicalactone on a copper chromite contact - can be converted into γ-valerolactone in 97% yield with the help of a catalyst system composed of laccase and 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO).
By selective hydrogenation of the double bond in the ring of α-angelicalactone - which can be produced in 90% yield by vacuum distillation of levulinic acid - with nanoscale palladium , γ-valerolactone is obtained in 98% yield.
The levulinic acid produced in the acid-catalyzed degradation of cellulose with the intermediate stages hexoses and hydroxymethylfurfural (in addition to equimolecular amounts of formic acid ) has recently become the most important starting material for γ-valerolactone as another platform molecule made from biomass.
The hydrogenation of levulinic acid to γ-valerolactone can be carried out with a variety of heterogeneous catalysts and catalyst systems, such as. B. nickel , platinum (IV) oxide , gold nanoparticles, ruthenium / tin catalysts, ruthenium nanoparticles, ruthenium- palladium - titanium dioxide -nanoalloys, with very good conversions of levulinic acid (> 90%) and excellent selectivities for γ-valerolactone (> 99%).
The hydrogenation can be carried out with hydrogen
or by reacting the formic acid obtained in equimolar amounts as a hydrogen source during the conversion of hydroxymethylfurfural together with levulinic acid at relatively low temperatures (100 ° C) with an exceptionally high yield (> 99%) and purity (> 99.9%).
Problems with the very different solubilities of starting materials and products can be countered by carrying out the hydrogenation in two-phase mixtures.
While the older work was based on hexoses and cellulose as the starting material for levulinic acid, the synthesis of γ-valerolactone directly from biomass , in particular from biomass of the so-called 2nd generation, i.e. cellulosic waste products, such as. B. Corn stover in the foreground.
Stereochemistry
| Enantiomers of γ-valerolactone | |
|---|---|
 ( R ) shape |
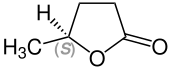 ( S ) shape |
γ-Valerolactone contains a stereocenter and is in the form of two mirror-image enantiomers . If no complex stereoselective synthesis method or separation technique has been used, a 1: 1 mixture ( racemate ) of ( R ) - and ( S ) -form is present.
A direct synthesis of the ( S ) -form is possible from levulinic acid with the help of a ligand- modified SEGPHOS - ruthenium - catalyst in methanol as co-solvent.
properties
Pure γ-valerolactone is a water-clear, colorless liquid with a pleasant odor, which is described as herbal, sweet, warm, tobacco-like, coconut-like and woody. The taste of γ-valerolactone is described as causing nausea. It solidifies at −31 ° C and boils at 205–206 ° C. Its flash point is 96 ° C. Γ-Valerolactone begins to decompose at temperatures above 170 ° C.
Because of its good solubility in water, the complete separation of GVL from aqueous solutions, as is usually the case in the acid degradation of hexoses, presents considerable difficulties.
Applications
γ-valerolactone is because of its simple and efficient accessibility from biomass, in particular from cellulose and lignocellulosic residues, such as. B. wood waste and food plant residues, discussed as a “green” solvent with a very wide liquid range of approx. 230 ° C, as a monomer for polyester and as a fuel additive or as a biogenic fuel (gasoline, diesel and kerosene).
In acidic mixtures of GVL and water, biomass in the form of lignocellulosic residues can be completely, i.e. H. including the lignin fraction, brought into solution and fed to a thermal saccharification. The soluble carbohydrates obtained can be extracted from the mixture in good yield by adding sodium chloride or liquid carbon dioxide .
Because of its low ring tension , the tendency towards ring-opening polymerization is only very slight in the case of γ-valerolactone. With ε-caprolactone , GVL was able to produce biodegradable polymers for biomedical applications, e.g. B. implants are copolymerized.
The disadvantage of using it as a fuel or as a diesel additive is the very low cetane number of γ-valerolactone of <10 (diesel> 50); advantageous is its property of significantly reducing particle emissions from diesel engines.
Because of its vomiting-irritating taste, γ-valerolactone appears to be unsuitable as a legal substitute for the party drug γ-butyrolactone , which is metabolized in the body to γ-hydroxybutyric acid (GHB), which is classified as an anesthetic . The γ-hydroxyvaleric acid produced from GVL is also significantly less effective than GHB.
Individual evidence
- ↑ a b c d e f g h i j Entry on gamma-valerolactone in the GESTIS substance database of the IFA , accessed on July 4, 2015(JavaScript required) .
- ↑ a b c data sheet γ-valerolactone from Sigma-Aldrich , accessed on July 4, 2015 ( PDF ).
- ↑ a b c d Entry on γ-valerolactone at TCI Europe, accessed on July 4, 2015.
- ↑ K. Yan, Y. Yang, J. Chai, Y. Lu: Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals . In: Appl. Catal. B: Environmental . tape 179 , 2015, p. 292-305 , doi : 10.1016 / j.apcatb.2015.04.030 .
- ↑ a b c D.M. Alonso, SG Wettstein, JA Dumesic: Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass . In: Green Chem. Band 15 , 2013, p. 584-595 , doi : 10.1039 / C3GC37065H .
- ↑ A. Messerschmidt: Investigations on the unsaturated acids. About allylacetic acid and valerolactone . In: Justus Liebigs Ann. Chem. Band 208 , no. 1–2 , 1881, pp. 92-104 , doi : 10.1002 / jlac.18812080107 .
- ↑ K. Komeyama, Y. Mieno, S. Yukawa, T. Morimoto, K. Takaki: Cationic iron-catalyzed addition of carboxylic acids to olefins . In: Chem. Lett. tape 36 , no. 6 , 2007, p. 752-753 , doi : 10.1246 / cl.2007.752 .
- ↑ JH Helberger, S. Ulabay, H. Civelekoglu: A simple process for the production of α-angelicalactone and the hydrogenative cleavage of oxygen-containing rings . In: Justus Liebigs Ann. Chem. Band 561 , no. 3 , 1949, pp. 215-220 , doi : 10.1002 / jlac.19495610307 .
- ^ A. Díaz-Rodríguez, I. Lavandera, S. Kanbak-Aksu, RA Sheldon, V. Gotor, V. Gotor-Fernández: From Diols to Lactones under Aerobic Conditions using a Laccase / TEMPO Catalytic System in Aqueous Medium . In: Adv. Synth. Catal. tape 354 , 2012, p. 3405-3408 , doi : 10.1002 / adsc.201200892 .
- ↑ Patent US2809203 : Method of converting levulinic acid into alpha angelica lactone. Applied on May 14, 1953 , published October 8, 1957 , Applicant: Heyden Newport Chemical Co., Inventor: RH Leonard.
- ↑ N. Kim, MS Kwon, CM Park, J. Park: One pot synthesis of recyclable palladium catalysts for hydrogenations and carbon-carbon coupling reactions . In: Tetrahedron Lett. tape 45 , no. 38 , 2004, p. 7057-7059 , doi : 10.1016 / tetlet.2004.07.126 .
- ↑ a b M.J. Climent, A. Corma, S. Iborra: Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuel . In: Green Chem. Band 16 , 2014, p. 516-547 , doi : 10.1039 / c3gc41492b .
- ↑ W. Wright, R. Palkovits: Development of heterogeneous catalysts for the conversion of levulinic acid into gamma-valerolactones . In: Chemsuschem . tape 5 , 2012, p. 1657-1667 , doi : 10.1002 / cssc.201200111 .
- ↑ Patent US2368366 : Process for the production of lactones. Filed August 21, 1942 , published January 30, 1945 , Applicant: Monsanto Chemical Co., Inventor: LP Kyrides, W. Groves, JK Craver.
- ↑ a b H.A. Schuette, RW Thomas: Normal valerolactone. III. Its preparation by the catalytic reduction of levulinic acid with hydrogen in the presence of platinum oxide . In: J. Am. Chem. Soc. tape 52 , no. 7 , 1930, p. 3010-3012 , doi : 10.1021 / ja01370a069 .
- ↑ X.-L. You, L. He, S. Zhao, Y.-M. Liu, Y. Cao, H.-Y. He, K.-N. Fan: Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts . In: Angew. Chem. Band 123 , no. 34 , 2011, p. 7961–7965 , doi : 10.1002 / anie.201100102 .
- ↑ a b D.M. Alonso, JMR Gallo, MA Mellmer, SG Wettstein, JA Dumesic: Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts . In: Catal. Sci. Technol. tape 3 , 2013, p. 925-931 , doi : 10.1039 / C2CY20689G .
- ↑ VS Java, M. Sudharkar, SN Kumar, A. Venugopal: Selective hydrogenation of levulinic acid to γ-valerolactone over a Ru / Mg-LaO catalyst . In: RSC Adv. Band 5 , 2015, p. 9044-9049 , doi : 10.1039 / C4RA16557H .
- ^ W. Luo, M. Sankar, AM Beale, Q. He, CJ Kiely, PCA Bruijnincx, BM Weckhuysen: High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone . In: Nature Commun. tape 5 , no. 6540 , 2015, doi : 10.1038 / ncomms7540 .
- ↑ V. Fabro, LT Mika, IT Horváth: Selective conversion of levulinic and formic acids to γ-valerolactones with the Shvo Catalyst . In: Organometallics . tape 33 , no. 1 , 2014, p. 181-187 , doi : 10.1021 / om400938h .
- ↑ SG Wettstein, DM Alonso, Y. Chong, JA Dumesic: Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as solvent in biphasic systems . In: Energy Environ. Sci. tape 5 , 2012, p. 8199-8203 , doi : 10.1039 / C2EE22111J .
- ^ V. Molinari, M. Antonietti, D. Esposito: An integrated strategy for the conversion of cellulosic biomass into gamma-valerolactone . In: Catal. Sci. Technol. tape 4 , no. 10 , 2014, p. 3626-3630 , doi : 10.1039 / c4cy00717d .
- ↑ József M. Tukacs, Bálint Fridrich, Gábor Dibó, Edit Székely, László T. Mika: Direct asymmetric reduction of levulinic acid to gamma-valerolactone: synthesis of a chiral platform molecule. In: Green Chemistry. 17, 2015, p. 5189, doi : 10.1039 / C5GC01099C .
- ^ The Good Scents Company: gamma-valerolactone
- ↑ a b H. Andresen-Streichert, H. Junge, A. Gehl, A. Müller, S. Iwersen-Bergmann: Uptake of Gamma-Valerolactone — Detection of Gamma-Hydroxyvaleric Acid in Human Urine Samples . In: J. Analyt. Toxicol. tape 37 , no. 4 , 2013, p. 250-254 , doi : 10.1093 / jat / bkt013 .
- ^ IT Horváth, H. Mehdi, V. Fábos, L. Boda, LT Mika: γ-Valerolactone, a sustainable liquid for energy and carbon-based chemicals . In: Green Chem. Band 10 , 2008, p. 238-242 , doi : 10.1039 / B712863K .
- ↑ JS Luterbacher, JM Rand, DM Alonso, J. Han, JT Youngquist, CT Maravelias, BF Pfleger, JA Dumesic: Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone . In: Science . tape 343 , no. 6168 , 2014, p. 277-280 , doi : 10.1126 / science.1246748 .
- ↑ M. Gagliardi, F. Di Michele, B. Mazzolai, A. Bifone: Chemical synthesis of a biodegradable PEGylated copolymer from ε-caprolactone and γ-valerolactone: evaluation of reaction and functional properties . In: J. Polym. Res. Band 22:17 , 2015, p. 1-12 , doi : 10.1007 / s10965-015-0661-2 .
- ↑ Á. Bereczky, K. Lukács, M. Farkas, S. Dóbé: Effect of γ-Valerolactone Blending on Engine Performance, Combustion Characteristics and Exhaust Emissions in a Diesel Engine . In: Natural Resources . tape 5 , 2014, p. 177–191 , doi : 10.4236 / no.2014.55017 .