α-angelical lactone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | α-angelical lactone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 6 O 2 | |||||||||||||||
| Brief description |
white, needle-like crystals or clear colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 98.10 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
52.3 Pa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.446-1.449 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
α-angelica is a dry distillation ( thermolysis ) of the platform chemical levulinic acid arising unsaturated alkylated γ-lactone , which as a flavoring and perfume , as a chemical intermediate and in attempts to enzyme induction of glutathione-S-transferase is used.
Occurrence and representation
α-Angelicalactone is found naturally in almonds, coffee, raisins, cranberries , coconuts, soybeans, as well as in white bread and liquorice .
The chemical synthesis, which was observed by Ludwig Wolff in 1883 and reported in detail in 1885, takes place by slow distillation of levulinic acid under normal pressure or by vacuum distillation of levulinic acid at temperatures of 150 to 175 ° C with elimination of water to give α-angelicalactone in 90% yield, with less than 5 % of the β-isomer (bp 205-208 ° C) are formed. The endothermic and acid-catalyzed reaction takes place via the so-called pseudolevulinic acid which is formed as an intermediate and, in addition to small amounts of the β-isomer, almost exclusively supplies α-angelicalactone.
Distillation of levulinic acid in the presence of concentrated phosphoric acid in vacuo gives the highest yield of α-angelicalactone with 95%.
The constitution of the α- and β-isomers of angelicalactone were proven by J. Thiele as early as 1901. Larger proportions of the β-isomer can be generated in the thermolysis of levulinic acid at higher temperatures.
An isomer of α-angelicalactone, also known as α'-angelicalactone (γ-methylene-γ-valerolactone) with an exocyclic double bond, can be obtained from 4-pentynoic acid with mercury (II) acetate in methylene chloride in 74% yield.
properties
The freshly distilled α-angelica lactone is a water-clear liquid that turns yellow after a few days at room temperature. The solid, which crystallizes out as long needles on cooling, sublimes at room temperature. The smell and taste of α-angelica lactone are described as sweet, oily, (coconut) nutty, coumarin and tobacco-like.
The lactone does not dissolve very much in water, but is readily soluble in many organic solvents. α-Angelicalactone isomerizes easily into the β-isomer, which because of its conjugated double bonds has a little higher stability.
As unsaturated dihydrofuranone, α-angelicalactone adds bromine to the corresponding dibromo-γ-valerolactone or hydrogen chloride to form monochloro-γ-valerolactone.
use
Hydrogenation to γ-valerolactone and 2-methyltetrahydrofuran
Hydrogenation of α-angelicalactone on a copper chromite contact at 150 ° C or in ionic liquids , such as. B. 1-Butyl-3-methylimidazolium hexafluorophosphate ([Bmim] PF 6 ) with a palladium on carbon (Pd / C) catalyst at room temperature provides γ-valerolactone with almost 100% selectivity when fully converted , as well as the solvent-free hydrogenation in a ruthenium on carbon (Ru / C) catalyst at normal pressure, which can be continued in a one-pot reaction with elimination of water to form 2-methyltetrahydrofuran (2-MTHF).
Both γ-valerolactone and 2-MTHF have recently been discussed as alternative biogenic fuels or fuel additives or as solvents.
The hydrogenation of α-angelicalactone at high temperatures (240 ° C) on copper chromite produces 1,4-pentanediol .
Levulinic acid ester by ring opening
From α-angelicalactone can be with alcohols with ring opening and catalysis with acidic ion exchangers , such. B. sulfonated polystyrene (Amberlyst 15) or sulfonated polytetrafluoroethylene ( Nafion ) represent esters of levulinic acid.
Instead of the ion exchange resins, insoluble and therefore easily separable, choline-modified polyoxometalates (heteropoly acids) can also be used. The esters of levulinic acid have also recently found interest as alternative biogenic fuels or fuel additives.
Amidation and decarbonylation
By reacting α-angelicalactone with primary amines in an aqueous medium and subsequent hydrogenation, 5-methyl-N-alkyl-2-pyrrolidones are accessible.
The reaction with methylamine produces 5-hydroxy-1,5-dimethyl-2-pyrrolidone in the first stage, from which 5-methylene-N-alkyl-2-pyrrolidone is formed with elimination of water, which is hydrogenated to the saturated pyrrolidone. Like N-methylpyrrolidone , which is mainly produced industrially from fossil raw materials , 5-methyl-N-methylpyrrolidone is suitable as an aprotic dipolar solvent for a variety of technical applications.
Decarbonylation of α-angelicalactone at 130–250 ° C in the presence of acidic silicate catalysts provides the versatile building block - z. B. for vitamin A - methyl vinyl ketone , which can be hydrogenated to the important ketone butanone .
Α-Angelicalactone dimers
When α-angelicalactone is heated in alkaline, e.g. B. with triethylamine , potassium hydroxide or with anhydrous potassium carbonate , almost quantitative (94%) dimers of angelicalactone are formed, which can be hydrogenated in high yield (88%) to branched C 7 to C 10 alkanes, which are discussed as gasoline substitutes .
Polymers with α-angelical lactone
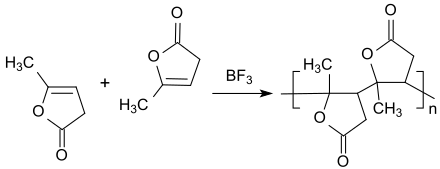
The unsaturated lactone α-angelicalactone can be viewed as a disubstituted vinyl acetate , which with strong Lewis acids , such as. B. boron trifluoride diethyl etherate , or by long UV irradiation on the (less reactive) double bond to sticky, dark red oligomers (n = 8-9) can be polymerized.
With polymerization initiators for free radical polymerization , such as. B. benzoyl peroxide or acetone peroxide , α-angelicalactone cannot be polymerized.
Like other lactones, e.g. B. caprolactone , α-angelicalactone with ring opening with basic catalysts, such as. B. sodium hydroxide or potassium tert-butanolate , to be polymerized to low molecular weight (M W to 20k) homopolymeric polylactones. The polymers are biodegradable in the soil within 180 days.
Relatively easily biodegradable copolymers of α-angelicalactone with styrene , caprolactam or methyl methacrylate with useful molecular weights and mechanical properties are also described.
Physiological effects of α-angelical lactone
Because of its taste and olfactory properties, α-angelicalactone is used as a flavor and fragrance in the food industry. In the US, α-angelica is a food additive GRAS ( English generally recognized as safe certified).
A tumor-inhibiting effect of α-angelicalactone was found in several animal studies by increasing the activity of the detoxifying enzyme glutathione-S-transferase and UDP-glucuronosyltransferase .
Individual evidence
- ↑ a b c G.A. Burdock: Fenaroli's Handbook of Flavor Ingredients, 6th Edition . CRC Press, Boca Raton, FL 2010, ISBN 978-1-4200-9077-2 , pp. 101 .
- ↑ a b data sheet alpha-Angelica lactone at AlfaAesar, accessed on April 15, 2017 ( PDF )(JavaScript required) .
- ↑ a b c d e f Entry on α-angelicalactone at TCI Europe, accessed on April 15, 2017.
- ↑ a b c d data sheet α-Angelica lactone from Sigma-Aldrich , accessed on April 15, 2017 ( PDF ).
- ↑ a b c d e f g L. Wolff: About some derivatives of levulinic acid . In: Justus Liebigs Ann. Chem. Band 229 , no. 3 , 1885, p. 249-285 , doi : 10.1002 / jlac.18852290302 .
- ↑ ANGELICA LACTONE ALPHA. (PDF; 83 kB) In: takasago.com. Takasago International Corp., accessed April 15, 2017 .
- ↑ a b c alpha-angelica lactone. In: thegoodscentcompany.com. The Good Scent Co., accessed April 3, 2017 .
- ↑ S. Young: About a new hepto- and octolactone . In: Justus Liebigs Ann. Chem. Band 216 , no. 1-2 , 1883, pp. 52 , doi : 10.1002 / jlac.18832160106 .
- ↑ Patent US2809203 : Method of converting levulinic acid into alpha angelica lactone. Applied on May 14, 1953 , published October 8, 1957 , Applicant: Heyden Newport Chemical Corp., Inventor: RH Leonard.
- ↑ RH Leonard: levulinic acid as a basic chemical raw material . In: Ind. Eng. Chem. Band 48 , no. 8 , 1956, pp. 1330-1341 , doi : 10.1021 / ie50560a033 .
- ↑ a b c Patent US7960592B2 : Production of methyl-vinyl ketone from levulinic acid. Filed January 12, 2010 , published June 14, 2011 , applicant: Wisconsin Alumni Research Foundation, inventor: JA Dumesic, RM West.
- ↑ a b c J.H. Helberger, S. Ulubay, H. Civelekoglu: A simple process for the production of α-angelicalactone and the hydrogenative cleavage of oxygen-containing rings . In: Justus Liebigs Ann. Chem. Band 561 , no. 3 , 1949, pp. 215-220 , doi : 10.1002 / jlac.19495610307 .
- ^ J.Thiele, R. Tischbein, E. Lossow: Ueber die Angelicalactone . In: Justus Liebigs Ann. Chem. Band 319 , no. 2 , 1901, p. 180-195 , doi : 10.1002 / jlac.19013190205 .
- ^ RA Amos, JA Katzenellenbogen: An efficient synthesis of .gamma.-methylene-.gamma.-butyrolactone (.alpha .'-angelicalactone). Application to the synthesis of deoxyobtusilactone and deoxyisoobtusilactone . In: J. Org. Chem. Band 43 , no. 4 , 1978, p. 560-564 , doi : 10.1021 / jo00398a007 .
- ↑ Patent US2761869 : Method of converting alpha angelica lactone into beta angelica lactone. Registered May 14, 1953 , published September 4, 1956 , Applicant: Newport Industries, Inc., Inventor: RH Leonard.
- ^ R. Cao et al .: Efficient conversion of α-angelica lactone into γ-valerolactone with ionic liquids at room temperature . In: ACS Sust. Chem. & Eng. tape 2 , no. 4 , 2014, p. 902-909 , doi : 10.1021 / sc4005185 .
- ↑ MG Al-Shaal, PJC Hausoul, R. Palkovits: Efficient, solvent-free hydrogenation of α-angelica lactone catalyzed by Ru / C at atmospheric pressure and room temperature . In: Chem. Commun. tape 50 , no. 71 , 2014, p. 10206-10209 , doi : 10.1039 / c4cc03970j .
- ↑ a b D.M. Alonso, SG Wettstein, JA Dumesic: Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass . In: Green Chem. Band 15 , 2013, p. 584-595 , doi : 10.1039 / C3GC37065H .
- ↑ MJ Climent, A. Corma, S. Iborra: Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuel . In: Green Chem. Band 16 , 2014, p. 516-547 , doi : 10.1039 / C3GC41492B .
- ^ V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María, AR Alcántara: 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry . In: Chemsuschem. tape 5 , no. 8 , 2012, p. 1369-1379 , doi : 10.1002 / cssc.201100780 .
- ↑ Patent US20060063948A1 : Preparation of levulinic acid esters from alpha-angelica lactone and alcohols. Applied on March 23, 2005 , published on March 23, 2006 , applicant: EI Du Pont de Nemours and Co., inventor: LE Manzer.
- ↑ X. Yi et al .: Synthesis of butyl levulinate based on α-angelica lactone in the presence of easily separable heteropoly acid catalysts . In: Chemsuschem. tape 10 , no. 7 , 2017, p. 1494-1500 , doi : 10.1002 / cssc.201601882 .
- ↑ C. Wedler, B. Costisella, H. Schick: Reactions of 4-oxoalkanoic acids. III. Synthesis of 5-methyl- and 5-methylenepyrrolidin-2-ones by reaction of α-angelica lactone with methylamine . In: J. Prakt. Chem. Volume 332 , no. 4 , 1990, pp. 557-562 , doi : 10.1002 / prac.19903320422 .
- ↑ Patent US2493373 : Dimers of angelica lactone. Filed July 31, 1946 , published January 3, 1950 , applicant: AE Staley Manufacturing Co., inventor: H. Wolff, WW Moyer.
- ↑ B. Lu et al .: Obtaining a high value branched bio-alkane from biomass-derived levulinic acid using RANEY® as hydrogenation catalyst . In: RSC Adv. Band 96 , no. 6 , 2016, p. 93956-93962 , doi : 10.1039 / C6RA14625B .
- ↑ M. Mascal, S. Dutta, I. Gandarias: Hydrodeoxygenation of the angelica lactone dimer, a cellulose-based feedstock: simple, high-yield synthesis of branched C7 - C10 gasoline-like hydrocarbons . In: Angew. Chem. Band 126 , no. 7 , 2014, p. 1885–1888 , doi : 10.1002 / anie.201308143 .
- ^ CS Marvel, CL Levesque: The structure of vinyl polymers. III. The polymer from α-angelica lactone . In: J. Am. Chem. Soc. tape 61 , no. 7 , 1939, pp. 1682–1684 , doi : 10.1021 / ja01876a013 .
- ↑ a b c V.E. Tarabanko, KL Kaygorodov: New biodegradable polymers based on α-angelica lactone . In: Chem. Sus. Dev. Band 18 , 2010, p. 321–328 ( sibran.ru [PDF]).
- ↑ T. Chen et al .: Degradable polymers from ring-opening polymerization of α-angelica lactone, a five-membered unsaturated lactone . In: Polym. Chem. Band 5 , no. 2 , 2011, p. 1190-1194 , doi : 10.1039 / C1PY00067E .
- ↑ VE Tarabanko, KL Kaygorodov: New environmentally benign polymers produced by copolymerization with α-angelica lactone . In: Macromol. Symp. Band 354 , no. 1 , 2015, p. 367-373 , doi : 10.1002 / masy.201400108 .
- ^ WA Nijhoff, GM Groen, WHM Peters: Induction of rat hepatic and intestinal glutathione-S-transferases and glutathione by dietary naturally-occuring anticarcinogens . In: Int. J. Oncol. tape 3 , no. 6 , 1993, pp. 1131-1139 , doi : 10.3892 / ijo.3.6.1131 .
- ^ WA Nijhoff, MA Bosboom, MH Smidt, WHM Peters: Enhancement of rat hepatic and gastrointestinal glutathione and glutathione-S-transferases by α-angelicalactone and flavone . In: Carcinogenesis . tape 16 , no. 3 , 1995, p. 607-612 , doi : 10.1093 / carcin / 3/16/607 .
- ↑ EMJ van der Logs, HMJ Roelofs, FM Nagengast, WHM Peters: Induction of rat hepatic and intestinal UDP glucuronosyltransferases by naturally Occurring dietary anticarcinogens . In: Carcinogenesis . tape 24 , no. 10 , 2003, p. 1651-1656 , doi : 10.1093 / carcin / bggl17 .