Carbonyldiimidazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Carbonyldiimidazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 6 N 4 O | |||||||||||||||
| Brief description |
crystalline colorless to yellowish solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 162.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
116-120 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,1'-Carbonyldiimidazole (also abbreviated to CDI ) is a colorless crystalline organic compound. It is used, among other things, as a reagent in peptide chemistry and in organic synthesis .
It is also known as the Staab reagent after Heinz A. Staab , who introduced it to organic synthesis in the 1950s.
Manufacturing
CDI is produced directly by reacting phosgene with four equivalents of imidazole under anhydrous conditions. After removal of the by-product imidazole hydrochloride and the solvent, CDI is obtained in a yield of about 90%.
In this reaction, imidazole acts as both a nucleophile and a base . During the hydrolysis of CDI, the imidazole is formed again with the elimination of carbon dioxide . The CDI content can be determined by determining the amount of carbon dioxide developed in this process.
properties
Carbonyldiimidazole forms colorless crystals that melt between 117 ° C and 122 ° C. Rapid hydrolysis occurs in water with the release of carbon dioxide . The connection is thermally unstable. A DSC measurement shows a strongly exothermic decomposition reaction from 186 ° C with an exothermicity of −507 kJ kg −1 or −82.2 kJ mol −1 .
Use in organic synthesis
CDI is mainly used in the conversion of alcohols and amines to the corresponding urea , carbamate or carbonic acid ester derivatives. CDI is a safe synthetic equivalent of phosgene because its toxicity is significantly lower.
Carboxylic acid derivatives
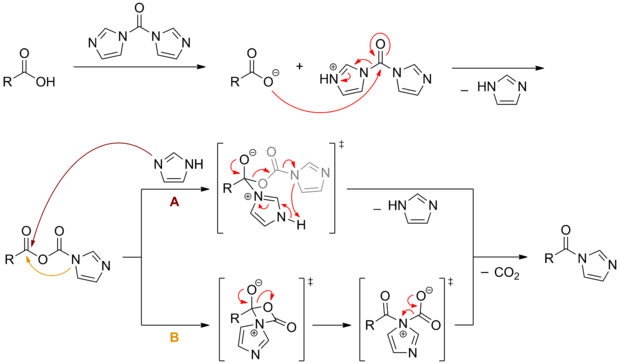
CDI can be used to activate carboxylic acids. First a mixed anhydride is formed , which then converts into an acylimidazole with the elimination of carbon dioxide . These activated species react in subsequent reactions like the corresponding carboxylic acid halides , but are much easier to handle and thus have a broader range of applications.
The mechanism of this transformation has not yet been finally clarified. After the mixed anhydride formation, two routes are discussed: (A) Intermolecular nucleophilic attack of a cleaved imidazole , followed by elimination of carbon dioxide and imidazole, or (B) Intramolecular nucleophilic attack of the "anhydride-imidazole" group, followed by ring opening and carbon dioxide -Elimination.
In the peptide synthesis, the carboxylic acid activated in this way is mixed with a suitable amino acid or peptide and the peptide extended by the corresponding amino acid is obtained with elimination of carbon dioxide and imidazole. The tendency of the amino acids to racemesize is low due to the mild conditions.
CDI can also be used for esterifications . Since the CDI adducts of carboxylic acids behave in a similar way to carboxylic acid halides, the product of the reaction of these with strong nucleophiles such as alcoholates is the corresponding ester. But the reaction with thiols and selenols are also known and lead to the corresponding sulfur or selenium analogues of an ester. If an acetal is used as the nucleophile, the corresponding glycoside is obtained .
Instead of the alcohol, a carboxylic acid can also be used as the nucleophile; the product is the corresponding anhydride. It is best - if the carboxylic acid is inexpensive - to use the carboxylic acid in double excess, since the insoluble or easily separable salt of the imidazole is then obtained as a by-product. If formic acid is used as a nucleophile, a potent formylation reagent is obtained.
Other reactions
When reacting with an ylide , a phosphonium salt is formed. In a further step, after deprotonation, these can be converted into an α, β-unsaturated carbonyl compound in a Wittig reaction.
The reduction with lithium aluminum hydride, on the other hand, provides the aldehyde and only a little alcohol or amine . In the reaction with Grignard reagents , the ketone is obtained in an analogous manner .
With acetylacetate anions , a substituted 1,3-diketone is obtained with the formation of a new carbon-carbon bond.
CDI can occur as a carbonyl equivalent in the synthesis of tetronic acids or pulvinones . For example, acetol ( hydroxyacetone ) reacts with CDI under basic conditions to form tetronic acid.
Individual evidence
- ↑ a b c d data sheet carbonyldiimidazole (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b c e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., entry for N, N'-Carbonyl Diimidazole, accessed November 4, 2019 .
- ↑ a b data sheet carbonyldiimidazole from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ^ Obituary by Heinz Staab by Thomas Carell, Francois Diederich, MPI
- ^ Staab: Synthesis, properties and preparative use of N, N'-carbonyl-di-imidazole , Angewandte Chemie, Volume 68, 1956, p. 754
- ↑ a b c d H. A. Staab: Syntheses Using Heterocyclic Amides (Azolides). In: Angew. Chem. Int. Ed. 1962 , 1 , pp. 351-367.
- ↑ a b Entry on 1,1'-carbonyldiimidazole. In: Römpp Online . Georg Thieme Verlag, accessed on November 4, 2019.
- ↑ HA Staab, K. Wendel: 1,1'-carbonyldiimidazole In: Organic Syntheses . 48, 1968, p. 44, doi : 10.15227 / orgsyn.048.0044 ; Coll. Vol. 5, 1973, p. 201 ( PDF ).
- ↑ a b A. Armstrong: N, N'-Carbonyldiimidazole. In: Encyclopedia of Reagents for Organic Synthesis 2001.
- ↑ Sperry, JB; Minteer, CJ; Tao, J .; Johnson, R .; Duzguner, R .; Hawksworth, M .; Oke, S .; Richardson, PF; Barnhart, R .; Bill, DR; Giusto, RA; Weaver, JD: Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing in Org. Process Res. Dev. 22 (2018) 1262-1275, doi : 10.1021 / acs.oprd.8b00193 .
- ↑ A. El-Faham, F. Albericio: Peptide Coupling Reagents, More than a Letter Soup In: Chem. Rev. 2011 , 111 , pp. 6557-6602; doi : 10.1021 / cr100048w .
- ^ R. Paul, GW Anderson: N, N'-Carbonyldiimidazole, a New Peptide Forming Reagent. In: J. Am. Chem. Soc. 1960 , 82 , pp. 4596-4600; doi : 10.1021 / ja01502a038 .
- ↑ H.-J. Gais: Synthesis of Thiol and Selenol Esters from Carboxylic Acids and Thiols or Selenols, Respectively. In: Angew. Chem. Int. Ed. 1977 , 16 , pp. 244-246.
- ↑ MJ Ford, SV Ley: A Simple, One-Pot, Glycosidation Procedure via (1-Imidazolylcaronyl) Glycosides and Zinc Bromide . In: Synlett 1990, pp. 255-256.
- ^ DW Brooks, et al : "C-Acylation under Virtually Neutral Conditions". In Angew. Chem. Int. Ed. 18 , 1979 pp. 72-74.
- ↑ PJ Jerris, et al. : "A Facile Synthesis of Simple Tetronic Acids And Pulvinones". In Tetrahedron Lett. 47 , 1979 pp. 4517-4520.