1,4-dioxan-2-one
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,4-dioxan-2-one | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 O 3 | |||||||||||||||
| Brief description |
white crystal lumps or liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 102.09 g mol −1 | |||||||||||||||
| Physical state |
solid or liquid |
|||||||||||||||
| density |
1.39 g cm −3 |
|||||||||||||||
| Melting point |
28 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
soluble in acetone , ethyl acetate and tetrahydrofuran |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,4-Dioxan-2-one ( p -dioxanone) is the lactone of 2- (2-hydroxyethoxy) acetic acid and, in contrast to the isomeric 1,3-dioxan-2-one - a cyclic carbonic acid ester (carbonate) - an intramolecular ester and an ether function. By ring-opening polymerization thereof is formed of poly ( p -dioxanone ), which is used as a biodegradable implant material.
Manufacturing
The common process for the production of 1,4-dioxan-2-one is the continuous gas phase dehydrogenation of diethylene glycol over copper or copper chromite catalysts at temperatures of up to 280 ° C.
Yields of up to 86% are achieved. The most complete possible separation of the excess diethylene glycol is critical for the suitability of the p-dioxanone as a monomer. The required purities of> 99.5% are achieved by recrystallization, vacuum distillation and melt crystallization or a combination of the methods.
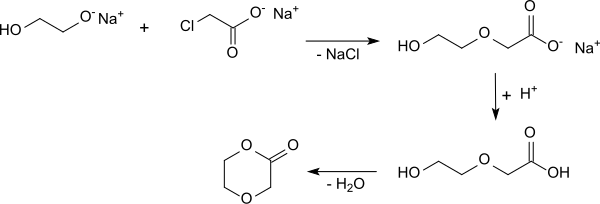
The sodium salt of 2- (2-hydroxyethoxy) acetic acid is formed from the monosodium salt of ethylene glycol and sodium monochloroacetate. After acidification with methanolic hydrochloric acid , concentration and separation of the sodium chloride formed, p-dioxanone is obtained in 67% yield in the vacuum distillation.
Another alternative is the oxidation of methyl diglycol (2- (2-methoxyethoxy) ethanol) to 2- (2-methoxyethoxy) acetic acid (3,6-dioxaheptanoic acid) with subsequent ether cleavage at the terminal methoxy group. The 2- (2- Hydroxyethoxy) acetic acid is cyclized with elimination of water to the lactone p-dioxanone.
properties
Pure 1,4-dioxan-2-one is a white crystalline solid that melts at 28 ° C. Even small amounts of impurities lead to a material that is liquid at room temperature and that dissolves in a number of solvents (ketones, esters, ethers, alcohols). B. can be recrystallized from ethyl acetate.
Applications
The oxidation of p- dioxanone with nitric acid or dinitrogen tetroxide produces diglycolic acid in yields of up to 75% .
In a ring-opening polymerization , the lactone 1,4-dioxan-2-one reacts under catalysis with organic tin compounds, such as. B. tin dioctoate (tin (II) -2-ethylhexanoate), dibutyltin dilaurate or with basic alkoxides, such as. B. aluminum isopropoxide to poly-1,4-dioxan-2-one,
a biodegradable, partially crystalline and thermolabile polymer for industrial and medical applications.
When heated above 100 ° C, depolymerization to the monomer p- dioxanone occurs.
Individual evidence
- ↑ a b c d Entry on 1,4-Dioxan-2-one at TCI Europe, accessed on March 30, 2015.
- ↑ a b c data sheet 1,4-dioxan-2-ones from Sigma-Aldrich , accessed on March 27, 2015 ( PDF ).
- ↑ a b c S.-W. Lee, S.-I. Kim, S.-J. Park: Solubility and density of p-dioxanone in organic solvent systems . In: J. Korean Oil Chem. Soc. tape 25 , no. 4 , 2008, p. 429-437 .
- ↑ a b Patent US2142033 : Process for the production of 2-p-dioxanone. Applied July 1, 1936 , published December 27, 1938 , Applicant: Carbide and Carbon Chemicals Corp., Inventor: RW McNamee, CM Blair.
- ↑ a b R.S. Bezwada, DD Jamiolkowski, K. Cooper: Poly (p-dioxane) and its copolymers, in Handbook of Biodegradable Polymers . Ed .: AJ Domb, J. Kost, DM Wiseman. Harwood Academic Publishers, 1997, ISBN 90-5702-153-6 , chap. 2 , p. 29-61 .
- ↑ a b Patent US5675022 : Recovery of dioxanone by melt crystallization. Applied on August 23, 1995 , published October 7, 1997 , applicant: Union Carbide Chemicals & Plastics Technology Corp., inventor: CG Moyers, MP Farr.
- ↑ Patent EP1138664A2 : Purified salt of β-hydroxyethoxy acetic acid, 2-p-dioxanone, and manufacturing method thereof. Applied March 30, 2001 , published October 4, 2001 , Applicants: Mitsui Chemicals, Inc., Inventors: S. Nakatani, T. Matsumoto, Y. Nakahara, H. Akieda, T. Ishitoku.
- ↑ Patent US5391768 : Purification of 1,4-dioxan-2-one by crystallization. Filed March 25, 1993 , published February 21, 1995 , applicant: United States Surgical Corp., inventor: Y. Jiang.
- ↑ Patent US3952054 : Process for preparing diglycolic acid. Applied December 5, 1974 , published April 20, 1976 , applicant: Monsanto Co., inventor: CY Shen.
- ↑ Patent US3645941 : Method of preparing 2-p-dioxanone polymers. Filed April 1, 1970 , published February 29, 1972 , applicant: Eastman Kodak Co., inventor: TC Snapp, AE Blood.
- ↑ Jean-Marie Raquez, Philippe Degée, Ramani Narayan, Philippe Dubois: Some Thermodynamic, Kinetic, and Mechanistic Aspects of the Ring-Opening Polymerization of 1,4-Dioxan-2-one Initiated by Al (O i Pr) 3 in Bulk . In: Macromolecules . tape 34 , no. 24 , October 2001, p. 8419-8425 , doi : 10.1021 / ma010396e .