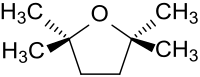
2,2,5,5-tetramethyltetrahydrofuran
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,2,5,5-tetramethyltetrahydrofuran | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 16 O | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 128.21 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.811 g cm −3 (20 ° C ) |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| boiling point |
112 ° C |
||||||||||||||||||
| Refractive index |
1.4050 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2,2,5,5-Tetramethyltetrahydrofuran is a derivative of tetrahydrofuran (oxolan) in which the hydrogen atoms on the two carbon atoms adjacent to the oxygen atom are replaced by two methyl groups each . Because of the lack of hydrogen atoms in the 2- and 5-positions, there is no peroxide formation by autoxidation in this cyclic ether .
TMO is seen as a “greener” alternative to hydrocarbon-based solvents such as B. n-hexane , cyclohexane and toluene are proposed.
Occurrence and representation
The mold Cladosporium cladosporioides CL-1 emits volatile organic compounds VOC, including 2,2,5,5-tetramethyltetrahydrofuran, which stimulate plant growth.
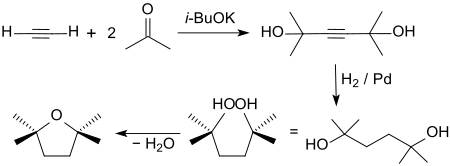
The chemical synthesis of 2,2,5,5-tetramethyltetrahydrofuran starts from 2,5-dimethylhexane-2,5-diol , which by hydrogenation on a palladium - aluminum oxide contact of 2,5-dimethyl-3-hexyne-2 , 5-diol (by Reppe ethynylation of acetone with acetylene ) is accessible in> 98% yield.
The cyclization to the furan derivative takes place in the presence of a beta zeolite at temperatures around 100 ° C practically quantitatively.
properties
2,2,5,5-Tetramethyltetrahydrofuran is a flammable, colorless liquid that is practically insoluble in water and, as an ether, does not form explosive peroxides even when exposed to UV radiation and in the absence of radical scavengers . Low polarity and relatively low boiling point make TMO a substitute candidate for more problematic hydrocarbons and ethers.
Applications
TMO as a synthesis raw material
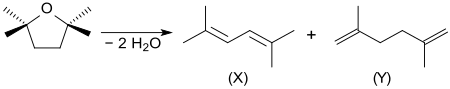
When 2,2,5,5-tetramethyloxolane is reacted with fluorine , all hydrogen atoms are replaced by fluorine to form perfluoro-2,2,5,5-tetramethyltetrahydrofuran. The two-fold elimination of water from TMO in acid gives a mixture of 2,5-dimethyl-2,4-hexadiene (X) and 2,5-dimethyl-1,5-hexadiene (Y).
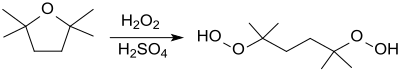
2,2,5,5-Tetramethyloxolane reacts with hydrogen peroxide in acid to form the corresponding tertiary dihydroperoxide , which can be used as a radical initiator for polymerization reactions or for the synthesis of other organic peroxides.
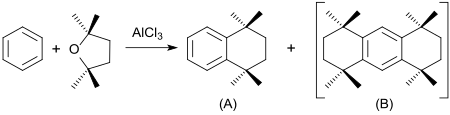
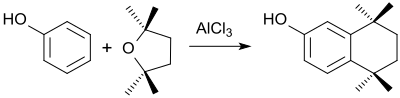
Friedel-Crafts alkylation of benzene in large excess with 2,2,5,5-tetramethyltetrahydrofuran in the presence of aluminum chloride produces 1,1,4,4-tetramethyltetrahydronaphthalene (A) in 85% yield in addition to the disubstitution product octamethyloctahydroanthracene (B).
The tetramethyltetrahydronaphthalene serves as the starting material for fragrances with a musky note.
With phenol , 5,5,8,8-tetramethyltetrahydro-2-naphthol is formed analogously, the retinoid- like secondary products of which are used for the treatment of skin diseases.
TMO as a solvent
The solvent properties of the cyclic ether 2,2,5,5-tetramethyloxolane are more similar to hydrocarbons, such as hexane, cyclohexane or toluene, than the cyclic ethers THF or 2-methyl-THF. The reason for this is the four methyl groups in the α position, which shield the ether oxygen strongly and also prevent the formation of peroxide, which is undesirable with ethers. Because of the steric hindrance of the methyl groups and the weak basicity of the ether oxygen, TMO is also a poorly binding complex ligand.
In model reactions such as B. in the radical copolymerization of butyl acrylate with acrylic acid , TMO shows a solvent behavior very similar to that of toluene.
The claim to offer a “greener” alternative to questionable aliphatic, cycloaliphatic and aromatic solvents cannot be met with the currently used synthetic route for TMO. The “green” route outlined, starting from hydroxymethylfurfural HMF via 2,5-dimethylfuran and 2,5-hexanedione , as well as its methylation to 2,5-dimethylhexane-2,5-diol, appears to be far too expensive and uneconomical. As long as the toxicology and ecology of 2,2,5,5-tetramethyltetrahydrofuran are based solely on predictions (“predicted low toxicity”), TMO does not meet the requirements for a replacement for hydrocarbon-based solvents made from fossil raw materials.
Individual evidence
- ↑ Data sheet 2,2,5,5-tetramethyltetrahydrofuran, 98% from Acros, accessed on December 15, 2019.
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 216 .
- ↑ a b c 2,2,5,5, -Tetramethyloxolane (TMO): An unusual ether which can replace hazardous hydrocarbon solvents. February 1, 2007, accessed December 15, 2019 .
- ↑ a b H.N. Huang, DF Persico, RJ Lagow, LC Clark, Jr .: Synthesis of unusual perfluorocarbon ethers and amines containing bulky fluorocarbon groups: New medial materials . In: J. Org. Chem. Band 53 , no. 1 , 1988, p. 78-85 , doi : 10.1021 / jo00236a016 .
- ↑ a b c data sheet 2,2,5,5-tetramethyltetrahydrofuran from Sigma-Aldrich , accessed on December 15, 2019 ( PDF ).
- ^ A b F. Byrne, B. Forier, G. Bossaert, C. Hoebers, TJ Farmer, JH Clark, AJ Hunt: 2,2,5,5-tetramethyltetrahydrofuran (TMTHF): a non-polar, non-peroxide forming ether replacement for hazardous hydrocarbon solvents . In: Green Chem. Band 19 , no. 15 , 2017, p. 3671-3678 , doi : 10.1039 / C7GC01392B .
- ^ D. Paul, KS Park: Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth . In: Sensors (Basel) . tape 13 , no. 10 , 2013, p. 13969-13977 , doi : 10.3390 / s131013969 .
- ↑ EL Jenner: α, α, α ', α'-Tetramethyltetramethylene Glycol In: Organic Syntheses . 40, 1960, p. 90, doi : 10.15227 / orgsyn.040.0090 ; Coll. Vol. 5, 1973, p. 1026 ( PDF ).
- ↑ Patent WO00150370 : Process for the production of acetylene alcohols and their secondary products. Applied on February 22, 2000 , published on August 31, 2000 , applicant: BASF AG, inventor: M. Brunner, J. Henkelmann, G. Kaibel, A. Kindler, C. Knoll, H. Rust, C. Tragut.
- ↑ a b Patent US20190284150A1 : Preparation of TMTHF. Registered on August 18, 2017 , published on September 19, 2019 , applicant: Nitto Belgium NV, inventors: G. Bossaert, C. Hoebers, B. Forier, F. Byrne, AJ Hunt, TJ Farmer, JH Clark.
- ^ A. Molnár, M. Bartók: Studies on the chemistry of diols and cyclic ethers - 52: Mechanism and stereochemistry of dehydration of oxolanes to dienes . In: Tetrahedron . tape 43 , no. 1 , 1987, pp. 131-141 , doi : 10.1016 / S0040-4020 (01) 89939-7 .
- ↑ Patent EP0513711A2 : Preparation of aliphatic dihydroperoxides. Applied on May 11, 1992 , published November 19, 1992 , Applicant: Air Products and Chemicals, Inc., Inventor: RM Machado, JA Marsella.
- ↑ Patent DE4426839A1 : Process for the production of 2,5-dimethyl-2,5-dihydroperoxy-hexane. Registered on July 28, 1994 , published on February 1, 1996 , Applicant: Peroxid-Chemie GmbH, Inventor: E. Hägel, W. Zeiß.
- ↑ Patent US4123469 : Process for production of bicyclic compounds. Registered June 30, 1977 , published October 31, 1978 , Applicant: International Flavors & Fragrances Inc., Inventor: MA Sprecker.
- ↑ Patent EP0337689B1 : Tetralin esters of phenols and benzoic acids having retinoid like activity. Filed April 10, 1989 , published March 10, 1993 , Applicant: Allergan, Inc., Inventor: RAS Chandraratna, RJ Weinkam.
- ↑ S. Krieck, P. Schüler, H. Görls, M. Westerhausen: Straightforward synthesis of rubidium bis (trimethylsilyl) amide and complexes of the alkali metal bis (trimethylsilyl) amides with weakly coordinating 2,2,5,5-tetramethyltetrahydrofuran . In: Dalton Trans. Band 47 , no. 36 , 2018, p. 12562-12569 , doi : 10.1039 / C8DT01539B .