2,4-dihydroxybenzoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
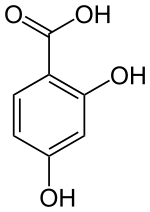
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 2,4-dihydroxybenzoic acid | |||||||||||||||||||||
| other names |
β-resorcylic acid |
|||||||||||||||||||||
| Molecular formula | C 7 H 6 O 4 | |||||||||||||||||||||
| Brief description |
colorless powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 154.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
208-211 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
2,4-Dihydroxybenzoic acid (β-resorcylic acid) is an aromatic compound that is derived from both benzoic acid and resorcinol (1,3-dihydroxybenzene). The structure consists of a benzene ring with an attached carboxy group (-COOH) and two hydroxyl groups (-OH) as substituents . It belongs to the group of dihydroxybenzoic acids .
presentation
2,4-Dihydroxybenzoic acid can be produced from resorcinol and potassium hydrogen carbonate by the Kolbe-Schmitt reaction .
Reactions and Derivatives
The bromination of 2,4-dihydroxybenzoic acid with elemental bromine in glacial acetic acid initially leads to 5-bromo-2,4-dihydroxybenzoic acid, which can be converted into 4-bromoresorcinol by decarboxylation . With further addition of bromine the 3,5-dibromo derivative is formed. If the latter is treated with nitric acid , the carboxy group and a bromine atom are split off, 4,6-dinitro-2-bromoresorcinol.
The nitration also initially takes place at position 5. If nitration is then continued with fuming nitric acid, a second nitro group attaches itself to position 3. Further treatment with nitric acid produces styphnic acid . With both mononitro and dinitro derivatives, the carboxy group can be removed by heating, resulting in 4-nitroresorcinol or 2,4-dinitroresorcinol . 5-Nitro-2,4-dihydroxybenzoic acid can be reduced to the corresponding amino compound with tin and hydrochloric acid .
When chlorine gas is introduced into a hot glacial acetic acid solution of 2,4-dihydroxybenzoic acid, the 5-chlorine derivative (CAS number: 67828-44-8, melting point 224-225 ° C) or, in excess of chlorine, the 3,5-dichloro derivative is formed (Melting point 229 ° C). This 5-chloro-2,4-dihydroxybenzoic acid condenses with resorcinols to give tetrahydroxybenzophenones, which react with ring closure to give the corresponding 7-chloro-3,6-dihydroxyxanthones. If 5-nitro acid is used instead of 5-chloric acid, the reaction with the ketone stops and ring closure does not take place.
When reacting with methyl iodide in methanol , initially only the para hydroxyl group is methylated, 2-hydroxy-4-methoxybenzoic acid is formed. The methylation of the second hydroxy group is difficult. The p-monoethyl ether can also be obtained with ethyl iodide .
When an aqueous solution of the sodium salt of 2,4-dihydroxybenzoic acid ( β-resorcylic acid ) is heated for a long time, partial isomerization to 2,6-dihydroxybenzoic acid ( γ-resorcylic acid ) takes place.
Orsellic acid is a close relative ; it differs specifically from 2,4-dihydroxybenzoic acid in that it has a 6-methyl group.
Web links
- Beilstein (System No. 1105), Vol. 10: H 377 , EI 176 , EII 251
- M. Kidney Stone, DA Clibbens: β-Resorcylic Acid In: Organic Syntheses . 10, 1930, p. 94, doi : 10.15227 / orgsyn.010.0094 ; Coll. Vol. 2, 1943, p. 557 ( PDF ).
Individual evidence
- ↑ a b Data sheet 2,4-dihydroxybenzoic acid from AlfaAesar, accessed on November 28, 2011 ( PDF )(JavaScript required) .
- ↑ Data sheet 2,4-Dihydroxybenzoic acid from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b c B. N. Mattoo: On the Complete Dissociation of 2: 4 Dihydroxy Benzoic (β-Resorcylic) Acid . In: Zeitschrift für Physikalische Chemie , 1959 , 22 (3–4), pp. 187–198 ( doi : 10.1524 / zpch.1959.22.3_4.187 ).
- ↑ Data sheet 2,4-dihydroxybenzoic acid from Acros, accessed on November 28, 2011.
- ↑ Free Online Encyclopedia: β-resorcylic acid , accessed January 31, 2017.
- ↑ a b Entry on 2,4-dihydroxybenzoic acid in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , pp. 351–352.
- ↑ S. v. Kostanecki: On the introduction of the carboxyl group in the phenols in: Reports of the German Chemical Society , 18 , pp. 3202-3206 (1885).
- ↑ RB Sandin, RA McKee: 4-Bromoresorcinol In: Organic Syntheses . 17, 1937, p. 23, doi : 10.15227 / orgsyn.017.0023 ; Coll. Vol. 2, 1943, p. 100 ( PDF ).
- ↑ F. v. Hemmelmayr: About some new derivatives of dioxybenzoic acids , in : months booklet for chemistry , 1912 , 33 , pp. 971–998 ( doi: 10.1007 / BF01552742 ).
- ↑ J. Zehenter: About some derivatives of α-dioxybenzoic acid , in: Monatshefte fur Chemie , 1881 , 2 , pp 468-483 ( doi: 10.1007 / BF01516530 ).
- ↑ a b F. v. Hemmelmayr: Via the action of nitric acid on β-resorcylic acid and some derivatives of the latter , in: Monatshefte fur Chemie , 1904 , 25 (1), pp 21-45 ( doi: 10.1007 / BF01540190 ).
- ↑ F. v. Hemmelmayr: About nitro derivatives of β-resorcylic acid [2,4-dioxybenzenecarboxylic acid ], in: Monatshefte für Chemie , 1905 , 26 (2), pp. 185-198 ( doi: 10.1007 / BF01532170 ).
- ↑ F. Hemmelmayr, T. Meyer: About the influence of different substituents on the adhesive strength of the carboxyl groups in substituted aromatic acids. Influence of a second carboxyl group and relative effect of chlorine and bromine , in : months booklet for chemistry , 1925 , 46 , pp. 143–156 ( doi: 10.1007 / BF01558962 ).
- ^ RB Sandin, RA McKee: Orientation in the Benzene Ring. The Preparation of 5-Chloro-β-resorcylic Acid , in: J. Am. Chem. Soc. , 1935 , 57 (6), pp. 1077-1078. doi: 10.1021 / ja01309a032 .
- ↑ R. Kurduker, NV Subba Rao: Search for Physiologically active compounds in Proceedings of the Indian Academy of Sciences - Section A 1963, 57 (5), pp 280-287. doi: 10.1007 / BF03049025 .
- ↑ a b F. Tiemann , A. Parrisius: About descendants of Resorcin , in: Reports of the German Chemical Society 1880 , 13 , pp. 2354-2381 ( full text ).
- ↑ S. v. Kostanecki, J. Tambor: Ueber die Constitution des Fisotins , in: Reports of the German Chemical Society 1895 , 28 , pp. 2302-2309 ( full text ).
- ↑ DK Hale, AR Hawdon, JI Jones and DI Packham: The carboxylation of resorcinol and the separation of β-and γ-resorcylic acid by ion-exchange chromatography , in: J. Chem. Soc. , 1952 , pp. 3503-3509 ( doi: 10.1039 / JR9520003503 ).





