2-acetyl nicotinic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-acetyl nicotinic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 7 NO 3 | ||||||||||||||||||
| Brief description |
pale beige solid or white needle-shaped crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 165.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| solubility |
soluble in water and in methanol , toluene , MTBE , ethyl acetate and n-butanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
As a six-membered heterocycle with a nitrogen atom, 2-acetylnicotinic acid is a derivative of pyridine which has a carboxy group in the 3-position . This with the trivial name nicotinic acid designated pyridine-3-carboxylic acid also has a 2-position acetyl group on. 2-Acetyl-3-pyridinecarboxylic acid is the starting material for the herbicide diflufenzopyr .
Occurrence and representation
The synthesis of 2 -acetylnicotinic acid was first described by Otto Rosenheim and Julius Tafel in 1893 as α-acetonicotinic acid. 6-Hydroxyquinoline (nowadays available in a modified Skraup synthesis as a one-pot domino reaction with Bamberger rearrangement in a simple manner and in good yield (77%) from nitrobenzene and glycerol ) was oxidized with chlorinated lime . After acidification, the oxidation product is converted into 2-ANA when heated to 140 ° C.
In the ozonolysis of 8-methylquinoline as the starting compound (by Doebner-Miller reaction from o-toluidine , sulfuric acid , sodium iodide and glycerol), 2-ANA is obtained in 70% yield. Less impurities due to by-products methylated on the pyridine ring are achieved when oxygen is passed through the reaction mixture after ozonolysis.
Because of the high risk of explosion, ozonolysis is obsolete as an industrial process for the production of 2-acetyl-3-pyridinecarboxylic acid.
An alternative is the synthesis route based on nicotinic acid (e.g. using a process from Lonza Group AG by oxidation of 5-ethyl-2-methyl-pyridine (MEP) using nitric acid) and oxidation to nicotinic acid N-oxide using hydrogen peroxide , the latter Reaction with acetic anhydride , subsequent deoxygenation of the N-oxide and ring cleavage to the end product 2-acetylnicotinic acid .
The pure product is obtained in total yields of 40%, based on nicotinic acid.
properties
In industrial synthesis, 2-acetylnicotinic acid is obtained as a beige-colored solid, which crystallizes as a pure substance in white needles. The compound is very easily soluble in hot water and can be recrystallized from it for cleaning. Alcohols, acetone and ethyl acetate, as well as toluene, MTBE and n- butanol also dissolve 2-ANA.
Applications
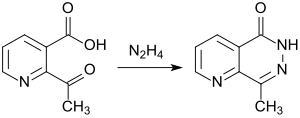
2-Acetylnicotinic acid reacts with hydrazine to form a bicyclic ring system of the pyrido-pyrazine type.
Functionalized pyrido [2,3-d] pyrazines have been synthesized and tested as potential herbicides.
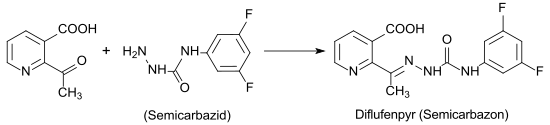
The main application of 2-acetylnicotinic lies in the synthesis of auxin transport - inhibitor diflufenzopyr that the effect of herbicides of the type of synthetic auxins such. B. Dicamba is used.
The semicarbazone derivative diflufenzopyr is approved in the USA and Canada, but not in the EU.
Individual evidence
- ↑ a b Patent US5098462 : Substituted semi-carbazones and related compounds. Applied on May 23, 1988 , published March 24, 1992 , Applicant: Sandoz Ltd., Inventor: RJ Anderson, MM Leippe, JT Bamberg.
- ↑ a b c O. Rosenheim, J. Tafel: Ueber die Oxydation des p-Oxychinolins . In: Ber. German Chem. Ges. Volume 26 , no. 2 , 1893, p. 1501-1513 , doi : 10.1002 / cber.18930260265 .
- ↑ H. Nagano, M. Hamana, Y. Nawata, S. Prachayasittikul, AN Abdel-Sayed, L. Bayer: Reinvestigation of the reaction of nicotinic acid 1-oxide with acetic anhydride . In: Heterocycles . tape 26 , no. 5 , 1987, pp. 1263-1270 , doi : 10.3987 / R-1987-05-1263 .
- ↑ a b c C. O'Murchu: Ozonolysis of quinolines: A versatile synthesis of polyfunctional pyridines . In: Synthesis . tape 11 , 1989, pp. 880-882 , doi : 10.1055 / s-1989-27423 .
- ↑ a b c Patent WO199967217 : Improved process for the production of substituted pyridinecarboxylic acids. Registered on June 4, 1999 , published on December 29, 1999 , applicant: DSM Fine Chemical Austria GmbH, inventors: G. Steinbauer, C. Zimmermann, E. Wressnegger, E. Steinwender.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2-acetylnicotinic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 17, 2020, is reproduced from a self-classification by the distributor .
- ↑ a b K. Paranjape, V. Gowariker, VN Krishnamurthy, S. Gowariker: The Pesticide Encyclopedia . CABI, Wallingford, UK 2015, ISBN 978-1-78064-014-3 , pp. 163 .
- ↑ H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel, C. Len: Toward the synthesis of 6-hydroxyquinoline starting from glycerol via improved microwave-assisted modified Skraup reaction . In: Catal. Commun. tape 44 , 2014, p. 15–18 , doi : 10.1016 / j.catcom.2013.07.029 .
- ↑ Patent DE1956117 : Process for the production of pyridinecarboxylic acids. Applied on November 7, 1969 , published on June 11, 1970 , applicant: Lonza AG, inventors: A. Stocker, O. Marthi, T. Pfammatter, G. Schreiner.
- ↑ EC Taylor, AJ Crovetti: Pyridine-1-oxide. I. Synthesis of some nicotinic acid derivatives . In: J. Org. Chem. Band 19 , no. 10 , 1954, pp. 1633-1640 , doi : 10.1021 / jo1375a012 .
- ^ BM Bain, JE Saxton: 1031. The reaction of nicotinic acid 1-oxide and 3-picoline 1-oxide with acetic acid . In: J. Chem. Soc. 1961, p. 5216-5223 , doi : 10.1039 / JR96100005216 .
- ↑ Moon MS, Lee SH, Cheong CS: A practical synthesis of nicotinic acid derivatives by palladium on charcoal . In: Bull. Korean Chem. Soc. tape 22 , no. 10 , 2001, p. 1167-1168 ( koreascience.or.kr ).
- ↑ NN Smolyar, YM Yutilov: Cyclo transformation in the series of fused 5-nitro-2 (1 H ) -ones . In: Russ. J. Org. Chem. Volume 44 , no. 2 , 2008, p. 274-281 , doi : 10.1134 / S1070428008020152 .
- ↑ Patent EP0555957A1 : Pyrido 2,3-d pyridazine derivatives as herbicides. Applied January 12, 1993 , published August 18, 1993 , Applicant: Rhone-Poulenc Agriculture Ltd., Inventor: R. Hewitt, SN Pettit, P. Smith.