Amifampridine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
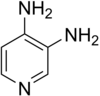
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Amifampridine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 7 N 3 | |||||||||||||||||||||
| Brief description |
light brown crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Mechanism of action |
Potassium channel blockers |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 109.13 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
216-218 ° C |
|||||||||||||||||||||
| solubility |
soluble in water (30 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Amifampridine is a drug from the group of reversible potassium channel blockers. a. Used to treat muscle weakness in Lambert-Eaton myasthenic syndrome (LEMS). Chemically, the substance can be assigned to the aminopyridines .
Clinical information
Approved field of application (indication)
Amifampridine is approved in the European Union (EU) and Switzerland for the symptomatic treatment of myasthenic Lambert-Eaton syndrome (LEMS) in adults.
Further possible areas of application
When it was made subject to a prescription requirement in Germany in 2008, a. the following possible areas of application:
- Presynaptic congenital myasthenia syndromes
- multiple sclerosis
- Spinal cord damage
- Disorders of the eye movements
- Calcium antagonist poisoning
- Botulism .
Efficacy for these areas of application cannot be derived from the literature, or only to a limited extent.
Drug interactions
Interactions with other drugs have not yet been systematically investigated.
Adverse effects (side effects)
The more common adverse effects include paresthesia , headache , dizziness , anxiety , fatigue , nausea, and sleep disorders . At higher doses, seizures , chorea, or myoclonus may occur.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Amifampridine reversibly blocks voltage-gated potassium channels. The active ingredient prevents potassium ions from leaving the nerve cells . Amifampridine therefore extends the depolarization at the presynaptic end of the nerve cells . The prolongation of the depolarization in turn indirectly increases the release of the messenger substance acetylcholine into the synapses and enables an improved muscle contraction .
toxicology
So far, amifampridine has only been examined toxicologically to a limited extent . Four-week studies on rats and dogs showed possible effects on the central nervous system , liver , kidneys , muscles and the conduction of stimuli at the atrioventricular node of the heart . Studies beyond four weeks and studies on possible effects on reproduction and tumor formation are lacking. Amifampridine is not genotoxic .
Other Information
History
Amifampridine has been used to treat LEMS and other neuromuscular diseases since the 1980s. The active ingredient was mostly used in the form of a free base .
Amifampridine was granted orphan drug status in the EU in 2002 . At the end of 2009 the company BioMarin ( Novato , USA ) received EU approval for the finished drug Firdapse . The active ingredient in Firdapse is the phosphate salt of amifampridine. The approval was granted under "exceptional circumstances" because the European approval authority assumed that the rarity of the disease LEMS did not enable the manufacturer to provide the usual information on efficacy, safety and tolerability. The approval was mainly based on information from specialist scientific literature.
price
In Germany, amifampridine is produced as an NRF 22.3 formulation in capsules with 5 milligrams (other strengths can also be produced) in pharmacies (costs here for 500 pieces with 5 milligrams 639 euros ) or as a finished drug Firdapse (costs here for 100 pieces with 10 milligrams 2893, 03 euros) prepared / dispensed in pharmacies after prescription by a doctor . For the maximum permitted dose of 60 mg per day, the annual costs for pharmacy preparations were mostly below 3,000 euros, while the annual costs for the finished medicinal product Firdapse are just under 64,000 euros when the maximum daily dose in Germany is taken. Similar price increases in the UK prompted well-known UK neurologists to send an open letter of protest to the UK Prime Minister .
Trade names
The trade name of the finished medicinal product authorized in the EU is Firdapse , after the name Zenas was initially intended; also commercialized in Switzerland under Firdapse .
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Amifampridine
Individual evidence
- ↑ a b Data sheet 3,4-diaminopyridine from Sigma-Aldrich , accessed on February 6, 2011 ( PDF ).
- ↑ a b c Entry on 3,4-diaminopyridine in the GESTIS substance database of the IFA , accessed on January 21, 2020(JavaScript required) .
- ^ Minutes of the results of the 60th meeting of the Committee of Experts on Prescription Requirements. BfArM , January 15, 2008, accessed March 6, 2017 .
- ↑ a b Firdapse 10 mg tablets. Summary of Product Characteristics . European Medicines Agency, December 23, 2009.
- ^ A b Assessment Report for Zenas. International Nonproprietary Name: Amifampridine . (PDF; 544 kB) European Medicines Agency, October 22, 2009, Procedure No .: EMEA / H / C / 001032; Retrieved February 6, 2011.
- ↑ New recipe form. Recipe information: 3,4-diaminopyridine and 4-aminopyridine. Federal Association of German Pharmacists' Associations. Govi, Eschborn 2009.
- ↑ ( Page no longer available , search in web archives: Firdapse 10 mg tablets. ) Red list online; accessed on February 5, 2011. Access only after registration.
- ↑ DJ Nicholl, D Hilton-Jones, J Palace et al: Open letter to prime minister David Cameron and health secretary Andrew Lansley. In: BMJ , 341, 2010, pp. C6466-c6466, doi: 10.1136 / bmj.c6466 , PMID 21081599 .