5-azacytosine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 5-azacytosine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 4 N 4 O | ||||||||||||||||||
| Brief description |
colorless powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 112.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.4572 g cm −3 at 25 ° C |
||||||||||||||||||
| Melting point |
> 350 ° C with decomposition |
||||||||||||||||||
| solubility |
slightly soluble in water (0.73 g cm −3 at 28 ° C ) |
||||||||||||||||||
| Refractive index |
1.8010 (20 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
5-Azacytosine is a base analogue of the nucleic base cytosine , whose CH group in the 5-position has been replaced by a nitrogen atom. The 5-azacytidines formed by linking with pentoses act as cytostatics .
Occurrence and representation
An older patent describes the strongly exothermic reaction to 2-amino-4-hydroxy-1,3,5-triazine by heating dicyandiamide DCD "over a small flame" with 100% formic acid . This creates 5-azacytosine as a white powder that does not melt up to 360 ° C.
If DCD is heated to 80 ° C. with excess pure formic acid, an exothermic reaction develops in which amidinourea formate is formed, which cyclizes to 5-azacytosine when heated dry to 150 ° C. (yield 82%).
4-Amino-1,3,5-triazin-2-one can also be obtained more gently by reacting guanylurea formate with triethyl orthoformate or dimethylformamide dimethylacetal as a cyclizing agent in dimethylformamide as a solvent in about 80% yield.
properties
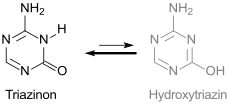
5-Azacytosine is a white, odorless, poorly water-soluble powder that can be recrystallized from water for cleaning. Aqueous solutions (0.7 g cm −3 ) have a pH value of 5.9 at 28 ° C. The compound is in the tautomeric equilibrium between 4-amino-1,3,5-triazin-2-one in the more stable amide form and 2-amino-4-hydroxy-1,3,5-triazine.
Applications
The synthetic nucleosides 5-azacytidine (5-azaC) - INN azacitidine - with the sugar residue β-D-ribofuranose and 5-aza-2'-deoxycitidine (5-azadC, decitabine ) with the sugar residue β- are derived from 5-azacytosine. D-deoxyribose .
In a modern synthesis variant , the reactive amino and hydroxyl groups are first protected by silylation with hexamethyldisilazane HMDS and the silylated 5-azacytosine with the acetyl- protected ribose (1,2,3,5-tetra-O-acetyl-β-D-ribofuranose) in The presence of trimethylsilyl trifluoromethanesulfonate TMSOTf converted to the protected 5-azacytidine. The trimethylsilyl - protecting group , in the work-up with sodium bicarbonate cleaved. The acetyl protective groups are split off with sodium methoxide in methanol MeOH to give crude 5-azacytosine, which is recrystallized with DMSO / MeOH to give the pure end product (37% yield).
Both antimetabolites inhibit the enzyme DNA methyltransferase , which reduces the formation of 5-methyl-2'-deoxycytidine (m5dC) in the genome , which in turn activates epigenetically silenced genes.
The 5-azacytosine derivative fazarabine (1-β-D-arabinofuranosyl-5-azacytosine, ara-AC) linked to the sugar residue β-D-arabinose showed insufficient effectiveness in phase II of the clinical trial and was discontinued.
Individual evidence
- ↑ a b c d e f g Safety Data Sheet: 4-Amino-1,3,5-triazin-2-one. Alzchem, July 21, 2019, accessed May 22, 2020 .
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 28 .
- ↑ M. Krečmerová, M. Otmar: 5-azacytosine compounds in medicinal chemistry: current stage and future perspectives . In: Future Med. Chem. Band 4 , no. 8 , 2012, p. 991-1005 , doi : 10.4155 / fmc.12.36 .
- ↑ Patent DE861384 : Process for the production of 2-amino-4-oxytriazine- (1,3,5). Published on October 29, 1941 , applicant: Deutsche Hydrierwerke AG, inventor: C. Grundmann.
- ↑ RC Hartenstein, I. Fridovich: Amidinourea formats, a precursor of 2-amino-4-hydroxy-s-triazine . In: J. Org. Chem. Band 32 , no. 5 , 1967, p. 1653-1654 , doi : 10.1021 / jo01280a095 .
- ↑ A. Piskala: Nucleic acid components and Their analogues. CI. Synthesis of 5-azacytosine (4-amino-1,2-dihydro-1,3,5-triazin-2-ones) and its methyl derivatives . In: Collect. Czech. Chem. Commun. tape 32 , no. 11 , 1967, p. 3966-3976 , doi : 10.1135 / cccc.19673966 .
- ↑ Patent CS269077B1 : Method of 5-azacytosine preparation. Registered on October 1, 1987 , published on April 11, 1990 , applicant: NN, inventor: A. Piskalá, A. Cihák, L. Korbová.
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry: Fundamentals, substance classes, reactions, concepts, molecular structure, 5th revised. Edition Thieme, Stuttgart 2005, ISBN 3-13-541505-8 , pp. 641 ( limited preview in Google Book search).
- ↑ Patent US7858774B2 : Synthesis of 5-azacytidine. Filed September 10, 2008 , published December 28, 2010 , applicant: Pharmion LLC, inventor: D. Ionescu, P. Blumbergs.
- ↑ SK Vujjini et al .: An improved and scalable process for the synthesis of 5-azacytidine: An antineoplastic drug . In: Org. Process Res. Dev. Band 17 , no. 2 , 2013, p. 303-306 , doi : 10.1021 / op300192e .
- ^ S. Schiffers, TM Wildenhof, K. Iwan, M. Stadlmeier, M. Müller, T. Carell: Label-free quantification of 5-azacytidines directly in the genome . In: Helvetica Chimica Acta . tape 102 , no. 3 , 2019, p. e1800229 , doi : 10.1002 / hlca.201800229 .
- ↑ J. Goffin, E. Eisenhauer: DNA methyltransferase inhibitors - state of the art . In: Ann. Oncol. tape 13 , no. 11 , 2002, p. 1699-1716 , doi : 10.1093 / annonc / mdf314 .