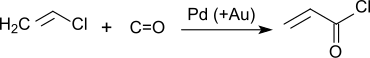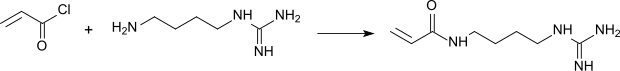Acryloyl chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
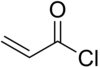
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acryloyl chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 3 ClO | |||||||||||||||
| Brief description |
colorless to light yellow liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 90.51 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure |
106.6 hPa at 20 ° C |
|||||||||||||||
| solubility |
soluble in chlorinated hydrocarbons such as dichloromethane and chloroform , in tetrahydrofuran |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acryloyl chloride is an α, β-unsaturated carbonyl compound and is particularly reactive as a chloride of acrylic acid or as a carboxylic acid chloride and functional alkene . Acryloyl chloride reacts violently with water with decomposition, and in contact with polymerization initiators it polymerizes explosively.
Because of its high toxicity, flammability and corrosiveness, acryloyl chloride must be handled with great care.
Manufacturing
When reacting acrylic acid with inorganic acid chlorides, such as. B. phosphorus oxychloride or thionyl chloride , or with organic compounds such as benzoyl chloride or benzotrichloride .
Acryloyl chloride can also be obtained by reacting vinyl chloride and carbon monoxide on a palladium - gold contact at temperatures around 150 ° C and pressures around 100 atm in batch and continuous mode.
The direct carbonylation of vinyl chloride with an excess of CO proceeds in very good yields (> 90% of theory) and, in the presence of alcohols as solvent, gives the corresponding acrylic acid ester .
properties
In its pure state, acryloyl chloride is a clear, colorless, volatile liquid with a pungent odor. The vapors are highly flammable and form explosive mixtures with air. In water, acryloyl chloride hydrolyzes in a strongly exothermic reaction to acrylic acid and hydrochloric acid and has a very toxic, caustic and corrosive effect.
Acryloyl chloride must therefore be stored in tightly closed, opaque containers in a dry place at 2-8 ° C and treated with effective amounts of a polymerization inhibitor, e.g. B. 100 ppm hydroquinone , 200 ppm hydroquinone monomethyl ether (MEHQ) or 200 ppm phenothiazine can be stabilized.
Applications
Because of its pronounced reactivity towards nucleophiles, acryloyl chloride is an effective agent for the formation of anhydrides, esters, thioesters and amides with the introduction of the polymerization-active acryloyl group.
Acryloyl chloride reacts with the alcohol 2-phenyl-2- (phenylthio) ethanol (obtained from thiophenol and styrene oxide in the presence of zinc chloride ) in yields of up to 94% of theory. to the corresponding acrylic acid ester monomer, which is characterized by a low glass transition temperature of 25 ° C and a high refractive index of 1.584.
The ester is suitable as a comonomer for the production of particularly thin, light and flexible contact lenses .
The acrylamidoagmatine obtained by reacting acryloyl chloride with agmatine is used as a comonomer for polymerization with acrylamide .
Hydrophilic gels for electrophoresis and chromatography can be produced from the strongly basic copolymers .
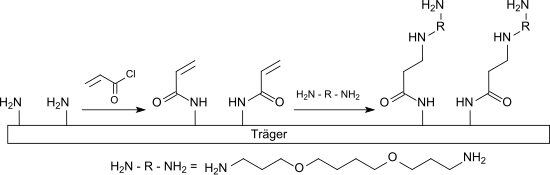
The ability of acrylic acid derivatives for Michael addition of nucleophiles, especially thiols and amines, to the activated double bond can, for. B. for the production of functionalized glass or silicon surfaces for the construction of microarrays for DNA and protein analysis.
For example, an amine-functionalized carrier surface is reacted with acryloyl chloride to form acrylamide and then with a hydrophilic long-chain diamine , such as. B. 1,4-bis (3-aminopropoxy) butane, linked in a Michael addition. The free primary amino group of this spacer molecule can with a bifunctional crosslinker such. B. phenylene diisothiocyanate (PDITC), for binding with an amino group-containing biomolecule, e.g. B. a protein, are activated.
The same synthesis strategy - conversion of activated acrylic acid derivatives such as acryloyl chloride with polyvalent amines and subsequent Michael addition - opens up access to hyperbranched polymers.
From acryloyl chloride and diethylenetriamine , a five-fold acylated intermediate is obtained [already referred to as hyperbranched polymer in the cited reference], which can further react to form a highly branched polymer.
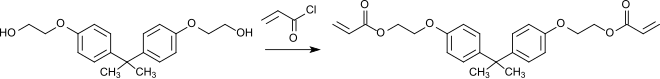
Acryloyl chloride reacts with Dianol 22 (bisphenol A bis (2-hydroxyethyl) ether) to form diacrylate (DDA), which is used as a crosslinker in coating resins because of its excellent film-forming and plasticizing properties.
The free radical homopolymerization of acryloyl chloride with azobis (isobutyronitrile) (AIBN) leads to poly (acryloyl chloride)
In 1,4-dioxane as solvent , at 50 ° C., with a yield of 90% of theory. Th. Unbranched polymer.
The corresponding side groups can be introduced into the polymer via polymer-analogous reactions of the acid chloride groups of the poly (acryloyl chloride) with amines or alcohols.
Individual evidence
- ↑ a b c d e f Entry on acrylic acid chloride in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e data sheet Acryloyl chloride 97% from Sigma-Aldrich , accessed on November 25, 2014 ( PDF ).
- ↑ a b c W.M. Haynes: Handbook of Chemistry and Physics, 91st ed., 2010-2011 . CRC Press, Boca Raton, 2010, ISBN 978-1-4398-2077-3 , pp. 3-442 .
- ↑ a b c Data sheet acrylic acid chloride (stabilized with phenothiazine) for synthesis (PDF) from Merck , accessed on November 25, 2014.
- ^ GR Deen: Solution Properties of Water-Soluble “Smart” Poly (N-acryloyl-N'-ethyl piperazine-co-methyl methacrylate) . In: Polymers . tape 4 , 2012, p. 32-45 , doi : 10.3390 / polym4010032 .
- ↑ W. Bauer: Acrylic Acid and Derivatives . In: Kirk-Othmer Encyclopedia of Chemical Technology . 5th edition. Volume 1. Wiley, 2004, ISBN 978-0-471-48522-3 .
- ^ GH Stempel Jr., RP Cross, RP Mariella: The preparation of acrylyl chloride . In: J. Am. Chem. Soc. tape 72 , no. 2 , 1950, p. 2299–2300 , doi : 10.1021 / yes.001161a527 .
- ↑ a b R.N. Jagtap, YT Chimankar: Synthesis of dumbbell shape hyperbranched polymer: Based on diethylene triamine and acryloyl chloride used as rheology modifier for water based paints . In: Adv. Appl. Sci. Res. Band 4 , no. 2 , 2013, ISSN 0976-8610 , p. 228-237 .
- ↑ Patent US5395966 : Process for the manufacture of acryloyl chloride. Applied on July 22, 1994 , published on March 7, 1995 , applicant: Atochem, inventor: J.-F. Croizy, P. Grosius.
- ↑ Patent US3626005 : Preparation of Unsaturated acyl Halides. Applied July 14, 1967 , published December 7, 1971 , Applicant: National Distillers and Chemical Corp., Inventor: JA Scheben, JM Fischer, IL Mador.
- ↑ Patent WO2014072995A2 : Sulfur containing high refractive index monomer. Filed October 30, 2013 , published May 15, 2014 , applicant: Council of Scientific and Industrial Research, India, inventor: S. Ponrathnam, RV Ghorpade, NN Chavan, KS Rajdeo, SS Bhongale.
- ↑ Patent US2007106090 : Method for synthesis of acrylamide derivatives. Filed October 18, 2004 , published May 10, 2007 , Applicant: GE Healthcare Bio-Sciences Corp., Inventors: M. Algotsson, P. Busson, N. Thevenin.
- ↑ K. Aboytes, J. Humphreys, S. Reis, B. Ward: A beginner's guide to microarrays, EM Blalock ed. Chapter 1: Slide coating and DNA immobilization chemistries. Kluwer Academic Publishers, 2003, ISBN 1-4020-7472-7 .
- ↑ C. Gao, D. Yan: Hyperbranched polymers: from synthesis to applications . In: Prog. Polym. Sci. tape 29 , 2004, pp. 183-275 , doi : 10.1016 / j.progpolymsci.2003.12.002 .
- ↑ External identifiers of or database links for bisphenol A bis (2-hydroxyethyl) ether : CAS number: 901-44-0, EC number: 212-985-6, ECHA -InfoCard: 100.011.805 , GESTIS- Substance database : 107723 , PubChem : 61909 , ChemSpider : 55770 , Wikidata : Q27116159 .
- ^ RH Leach, RJ Pierce (Eds.): The Printing Ink Manual . 5th edition. Springer, 2007, ISBN 0-948905-81-6 , pp. 286 .
- ↑ RC Schulz, P. Elzer, W. Kern: About the polymerization of acrylic acid chloride . In: Makromol. Chem. Band 42 , no. 1 , 1960, p. 189–196 , doi : 10.1002 / macp.1960.020420119 .
- ^ HR Kricheldorf, O. Nuyken, G. Swift (Ed.): Handbook of Polymer Synthesis . 2nd Edition. M. Dekker, 2005, ISBN 0-8247-5473-5 , pp. 286-288 .