adsorption
As adsorption (from latin adsorpio from adsorbere "(an) suck") refers to the enrichment of substances from gases or liquids at the surface of a solid body, more generally at the interface between two phases .
This differs from absorption , in which the substances penetrate into the interior of a solid or a liquid. The generic term for adsorption and absorption is sorption .
If two or more species adsorb on a surface, this is called coadsorption . This is particularly important in the case of catalysts , in which the different types of atoms are adsorbed on the same surface and react there.
Demarcation
In the general sense, adsorption is a physical process in which substances (usually molecules) stick to the surface of another substance and accumulate on its surface. The forces that cause attachment are not chemical bonds , just Van der Waals forces . Hence, this form of adsorption is more accurately called physical adsorption or physisorption . The physical adsorption principle also includes the reverse process, the desorption , as a system always strives for a balance between the adsorption and desorption of a substance. The surface on which adsorption takes place can be the surface of a solid or the surface of a liquid. In general, the surfaces are called interfaces . The phase from which the substance reaches the interface can be a gas phase or a liquid ( solution ). The particles reach the interface through their undirected, thermally driven molecular movement . However, the following sections mainly describe the adsorption of gases onto solid surfaces. The physisorption is next to the Ab sorption an everyday process and takes place on almost all surfaces that gases (air) or liquid (water) temperatures.
In the special case of chemical adsorption , also known as chemisorption , substances are bound to the surface of a solid by chemical bonds. Chemisorption can have considerable consequences for the adsorbed substance and is therefore different from physisorption. By breaking and forming chemical bonds, other substances can form and a desorbed substance (desorbate) can be a product of a chemical reaction. As a rule, high reaction heats occur in chemisorption, which can be used as a criterion for distinguishing between physisorption and chemisorption. Chemisorption is often not an equilibrium reaction, i.e. irreversible, may only take place at high temperatures and often does not lead to an accumulation of substances on the surface. Chemisorptions are important steps that occur in heterogeneous catalysis processes.
In general, the term adsorption should only be used for the everyday processes of physisorption . Special adsorptions with chemical processes should be explicitly referred to as chemisorption .
Physical adsorption
| Physical adsorption | |
| Monolayer | Adsorption energy |

|

|
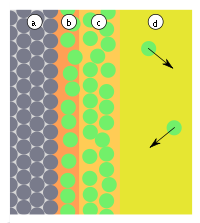
| Fig.1: a) adsorbent, b) adsorbate at the interface, c) gas phase or solution with adsorptive | Fig.2: a) adsorbent, b) adsorbate, c) gas phase, d-distance, E-energy, E b -adsorption energy, (1) reflection, (2) adsorption |
| Monolayer and condensate | selectivity |
|

|
| Fig.3: a) adsorbent, b) adsorbate, c) condensate d) gas phase or solution with adsorptive | Fig.4: a) adsorbent, b) adsorbate, c) gas phase or solution with different adsorptive: Molecules in blue are preferentially adsorbed here |
| Rough surface | Inner surface |

|

|
| Fig.5: a) Adsorbent with rough surface b) Adsorbate, c) Gas phase or solution with adsorptive | Fig.6: Structure of zeolite A with a large inner surface |
The physical adsorption ( physisorption ) of a substance on a surface is similar to a chemical equilibrium reaction. However, the adsorbed substance (adsorbate) does not form a chemical bond with the surface , but adheres due to weaker forces similar to adhesion . As a rule, only Van der Waals forces occur. The adsorption energy in physisorption is in the range from 4 to 40 kJ / mol. Chemical bonds within an adsorbed particle remain, but become polarized . For this equilibrium reaction, one can set up a reaction equation between the substance that is adsorbed (adsorptive), the surface of the solid (adsorbent) and the adsorbate:
The following applies:
- - Particles in the gas phase or in solution (adsorptive)
- - particles on the surface (adsorbate)
- - Free surface areas of the adsorbent
If particles hit the surface (Fig. 1), they are either reflected or adsorbed. Adsorption occurs when the particles can give up their kinetic energy and the adsorption energy ( E b ), which is released in the form of heat (Fig. 2). The reaction is therefore exothermic . The adsorption is reversible, since the particles can leave the surface with a similar amount of energy, since these reactions are usually not inhibited by transition states. The activation energy ( E a ) of the reaction is therefore equal to the adsorption energy ( E b ). Adsorbed particles do not have a fixed binding site on the surface, do not linger at the point where they were adsorbed, but move freely along the surface.
The position of the equilibrium depends on the properties and size of the surface, the properties and pressure (or concentration) of the adsorptive, as well as the temperature. At 300 ° C and normal pressures, the equilibrium is often all the way to the left. If the equilibrium is on the right side, the particles from the gas phase ideally form a monomolecular layer on the surface.
In practice, further layers can form after the formation of the monolayer (Fig. 3). Strictly speaking, one only counts the formation of the first layer for adsorption, since only here interactions between the surface and the adsorptive act. In the other layers there are only interactions between the adsorbates. Strictly speaking, it is then a matter of condensation of the adsorptives. The heat of condensation (binding energy of the adsorbates to one another) is usually significantly lower than the adsorption energy of an adsorbate to the adsorbent.
As a rule, the surfaces of the adsorbents do not have an ideally flat structure, but rather have numerous defects ( lattice defects ). These defects form places where the adsorbate is particularly tightly bound (Fig. 5). The mobility of the adsorbate allows these sites to be occupied regardless of the site where the adsorptive was adsorbed. The efficiency of an adsorbent therefore increases with the number of its defects. For this reason, the largely amorphous activated carbon is a particularly powerful adsorbent compared to graphite . Other high-performance substances such as zeolites are not amorphous, but have a very narrow channel system (Fig. 6), a so-called inner surface , in which only a few molecules (adsorptives) can be placed next to each other. With such substances, adsorption and condensation can hardly be distinguished. Occasionally, such processes are also called capillary condensation (see capillarity ).
Under normal conditions, surfaces are always covered by substances. Solvent molecules sit on the surface of solids in contact with solvents. Surfaces in contact with air are e.g. B. occupied with water molecules. Practically suitable adsorbents are characterized by selectivity (Fig. 4). Dissolved substances from solutions displace the solvents and certain substances from the air displace the water from the surface, as they are more strongly bound ( adsorption displacement ). This accumulation of certain substances on the surface allows the production of gas filters (e.g. in respiratory protection filters ) and processes of adsorption chromatography .
Adsorption isotherm
| Adsorption isotherm | |
| monomolecular layer | |
|
|
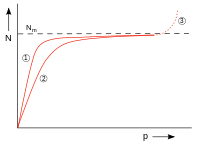
| Fig. 7: N: number of adsorbed particles, p: pressure, N m : number of particles when the surface is fully covered, (1) and (2) adsorption isotherms for two temperatures; higher temp. with (2), (3) condensation | |
| Capillary condensation | |

|
|
| Fig. 8: Adsorption behavior of large-pore / coarse-pored materials such as silica gel | |
A degree of surface coverage can be defined for the adsorption of monomolecular layers :
- , With
- : Number of molecules adsorbed
- : Number of molecules when fully occupied
The degree of occupancy in a gas - solid surface system depends on the partial pressure of the gas and the temperature .
To characterize (measure) the surfaces of substances, so-called adsorption isotherms are preferably recorded, at which the temperature is kept constant. Gases such as nitrogen or noble gases are often used as adsorbates. The position of the equilibrium of a system is then only dependent on the pressure.
A practically important isotherm is the Langmuir isotherm , which can be represented in this form:
The size can be viewed as the ratio of the rate constants of desorption and adsorption of a system. Figure 7 shows the course of occupancy. For small ( ) the adsorption isotherm is proportional to . For large ( ) the curve approaches the monomolecular occupancy . Condensation occurs at higher pressures, but this is not described by the Langmuir isotherm.
In addition to Langmuir's adsorption isotherm , chemical adsorption can also be described with Freund's equation .
Materials with a rough or internal surface often show a different adsorption behavior, which cannot be described by Langmuir isotherms. The isotherm according to Brunauer, Emmett and Teller ( BET isotherm ) is often used :
- , with a as a system-specific constant.
Adsorption isotherms of materials such as silica gel show a steep increase at low pressure (Fig. 8), but only tend to converge and exceed this value early. This is where capillary condensation comes into play, which is energetically close to the heat of adsorption of a monomolecular occupancy.
Competing adsorption
If molecules of one component occupy the surface of an adsorbent that was intended for the separation of another component, this is referred to as competitive adsorption. This is important for technical separation tasks, since in many cases mixtures of substances occur (e.g. a hydrocarbon and water vapor). In such cases, the selectivity of the adsorbent is of great importance. Competing adsorption can be observed and quantified by measuring breakthrough curves with two or more adsorptives.
Chemical adsorption
| Chemical adsorption | |||||||||||||||
| Monolayer | |||||||||||||||

|
|||||||||||||||
|
Fig. 9: a) adsorbent, b) adsorbate, c) gas phase with adsorptive, (1) physisorption, (2) dissociative chemisorption (3) directed chemisorption |
|||||||||||||||
| Adsorption energy | |||||||||||||||
|
|||||||||||||||
The chemical adsorption ( chemisorption ) is an accumulation of substances on the surface of solids like the chemical bonding forces. While the binding forces act up to approx. 40 kJ / mol in physical adsorption, the heats of reaction in chemical adsorption are in the range from 40 to 420 kJ / mol. During chemisorption, bonds are made to the surface and the electronic structure within the adsorbate is changed. Due to the direct bonds to the surface, a maximum of monomolecular layers can form. In spite of a chemical bond to the surface, the adsorbate can mostly move along the surface - as with physisorption.
Chemisorption can have significant consequences for the adsorbed substance, which depend on the properties of the adsorbate, the adsorbent and the temperature of the system. Chemisorption is a fundamental process in solid catalyst catalysis, known as heterogeneous catalysis . Bonds within the adsorbed substance can be weakened. Chemical reactions with other reaction partners can now proceed more easily; one speaks of a catalytic activation of the reaction.
Under certain circumstances, bonds within the adsorbate can be broken, which can lead to the adsorbate breaking down into two or more particles ( dissociative chemisorption ). For example, B. Hydrogen (H 2 ) on metals such as iron, platinum and palladium to form individually bonded hydrogen atoms (Fig. 9, (2)). Dissociative chemisorptions can result in particles being desorbed other than those that were adsorbed. Chemisorption then triggered ( catalyzed ) a chemical reaction .
Carbon monoxide (CO) only dissociates at high temperatures when adsorbed onto the above-mentioned metals. As a rule, so-called directed chemisorption takes place: CO binds to the metal surface via the carbon atom (Fig. 9, (3)), analogous to the direction of the bond that occurs with metal carbonyls .
Adsorption is an exothermic reaction, which, however , can take place in transition states that are unfavorably high in terms of energy (activated adsorption). With activated adsorption - in contrast to physisorption - adsorption only takes place at higher temperatures and the speed increases with increasing temperature.
In contrast to physisorption, the activation energy of desorption is often unequal to the activation energy of adsorption. The activation energy of the desorption results from the changes in the adsorbate. While some adsorbed particles (e.g. H 2 or O 2 ) are dissociated, other particles (e.g. CO 2 ) have changed their spatial configuration. The size of the activation energies and the heat of adsorption depend not only on the type of adsorbed particles or their reaction products, but also on the chemical composition and the (electronic) structure of the adsorptive (table “Adsorption energy”). In the case of chemisorption, the adsorption can be irreversible if an (too) high activation energy is required for the desorption of certain particles. If such chemisorption occurs with catalysts, this leads to deactivation and one speaks of catalyst poison .
Applications
Physisorption
As heat is released during adsorption and heat is absorbed during desorption, heat pumps ( adsorption chillers ) based on adsorption are built. During the operation of adsorption chillers, the physical state of the fluid is changed.
Physisorption in the gas phase
As adsorbents , for example, come activated carbon , activated coke , silica gel or molecular sieves ( zeolites ) in the form of beds or in a structured form for practical use. Gas filters such as respiratory protection filters and processes for drying moist air ( adsorption drying ) are based on physisorption .
Adsorptive processes are often used to clean exhaust gases . They are used to retain hydrocarbons such. B. in the form of solvents or gasoline vapors . Adsorption processes are also used to remove odorous substances, hydrogen sulfide and sulfur dioxide. Adsorption is also used to smooth concentration peaks in front of oxidation catalysts.
Many processes for the separation or purification as well as the analysis of substance mixtures are based on chromatographic methods , the adsorption chromatography . All of these methods use the substance-specific equilibrium reaction of physisorption between an interface and a mobile phase .
In the case of technical separation and of gas mixtures, physisorption is used, since gases accumulate to different degrees on the surface of an adsorbent, depending on pressure and temperature. A distinction is made between the operating modes of temperature change and pressure change adsorption . For example, high-purity hydrogen can be obtained from synthesis gas . Another application for the technical separation of gas mixtures is the removal of CO 2 from biogas .
Physisorption in the liquid phase
For the purification of many natural substances, and nowadays especially in water treatment , wastewater treatment , groundwater remediation and in industrial water management , adsorption is used to remove harmful substances in water or to recover substances.
Chemisorption
Chemisorption is the fundamental process in heterogeneous catalysis . Catalysts are suitable solids for this. Hydrogen is used for the hydrogenation of C = C double bonds and Raney nickel or nickel-aluminum alloys are used as the catalyst . The chemisorption of hydrogen on catalysts also plays an important role in the Haber-Bosch process for the production of NH 3 . The chemisorption of carbon monoxide and hydrogen enables the synthesis of hydrocarbons in the Fischer-Tropsch synthesis . Also automotive catalysts based on a heterogeneous catalysis.
literature
- Wilhelm Schwieger, Helge Toufar: Inorganic-porous materials: preparation, processing and industrial production . Springer, Berlin 2007, ISBN 978-3-540-23649-8 .
- Dieter Bathen, Marc Breitbach: Adsorption technology . Springer, Berlin 2001, ISBN 978-3-540-41908-2 .
- LW Bruch, Milton W. Cole, Eugene Zaremba: Physical Adsorption: Forces and Phenomena . Dover Pubn Inc, 2007, ISBN 978-0-486-45767-3 .
- Stephen Brunauer: The Adsorption of Gases and Vapors Vol I - Physical Adsorption . Dodo Press, 2007, ISBN 978-1-4067-5030-0 .
- VDI 3674: 2013-04 Exhaust gas cleaning through adsorption; Process gas and waste gas cleaning (Waste gas cleaning by adsorption; Process gas and waste gas cleaning). Beuth Verlag, Berlin. ( Summary and table of contents online )
Individual evidence
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition. Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , p. 1026.
- ↑ R. Brdicka; In: Fundamentals of Physical Chemistry ; VEB Deutscher Verlag der Wissenschaften, Berlin 1958, p. 482.
- ↑ VDI 3674: 2013-04 Exhaust gas cleaning through adsorption; Process gas and waste gas cleaning, pp. 14–15.
- ^ Römpp Lexikon Chemie
- ↑ Heinz Kuschel: Adsorptive removal of a solvent mixture from the exhaust air of a film processing company . Dust - cleanliness. Luft , ISSN 0949-8036 , 36 (1976) No. 7, pp. 303-306.
- ↑ VDI 3674: 2013-04 Exhaust gas cleaning through adsorption; Process gas and waste gas cleaning, pp. 51–62.
- ↑ Rolf Germerdonk: Adsorption of odorous and other problematic substances . Dust - cleanliness. Luft, ISSN 0949-8036 , 36 (1976) No. 7, pp. 306-311.
- ↑ Kurt Hedden , L. Huber, BR Rao: Adsorptive cleaning of exhaust gases containing hydrogen sulfide . Dust - cleanliness. Luft, ISSN 0949-8036 , 36 (1976) No. 7, pp. 313-317.
- ↑ Karl Knoblauch, R. Noack: Operating experience with the mining research process for flue gas desulfurization . Dust - cleanliness. Luft, ISSN 0949-8036 , 36 (1976) No. 7, pp. 318-323.
- ↑ VDI 3476 sheet 2: 2010-01 exhaust gas cleaning; Process of catalytic exhaust gas purification; Oxidative processes (Waste gas cleaning; Catalytic waste gas cleaning methods; Oxidative processes). Beuth Verlag, Berlin, p. 28.
- ↑ VDI 3896: 2015-10 Emission Reduction; Preparation of biogas to natural gas quality (Emission Control; Preparation of biogas to natural gas quality). Beuth Verlag, Berlin, p. 15.
- ^ Otto Westphal , Theodor Wieland , Heinrich Huebschmann: life regulator. Of hormones, vitamins, ferments and other active ingredients. Societäts-Verlag, Frankfurt am Main 1941 (= Frankfurter Bücher. Research and Life. Volume 1), pp. 118–122.
- ^ Gabi Förtsch, Heinz Meinholz: Manual of operational pollution control . 2013, Springer Spectrum, ISBN 978-3-658-00005-9 , pp. 286-293.
















![N = {\ frac {N _ {\ mathrm {m}} ap} {(p_ {0} -p) [1+ (a-1) p / p_ {0}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/acbcb4adddb1220b2d5eefcbc1a2ca3fa9045623)