Aluminum bromide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
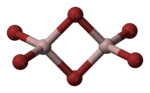
|
|||||||||||||||||||
| Aluminum bromide dimer: __ Al 3+ __ Br - |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum bromide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | AlBr 3 | ||||||||||||||||||
| Brief description |
shiny, colorless leaflets |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 266.69 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.2 g cm −3 |
||||||||||||||||||
| Melting point |
97.5 ° C |
||||||||||||||||||
| boiling point |
263 ° C |
||||||||||||||||||
| Vapor pressure |
1.3 h Pa (81 ° C) |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−516 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum bromide is an inorganic chemical compound made from bromine and aluminum with the empirical formula AlBr 3 .
Extraction and presentation
Aluminum bromide can be synthesized by passing bromine vapor over a glowing mixture of carbon and aluminum oxide or by the direct action of bromine on aluminum.
properties
The molar mass is 266.69 g / mol, its density 3.2 g / cm³. It has a melting point of 97.5 ° C and a boiling point of 263 ° C.
In the solid state, aluminum bromide forms Al 2 Br 6 molecules, in which two bromide ions appear as bridging ligands , causing the aluminum atom to reach an electron octet.
Aluminum bromide is soluble in benzene , toluene , carbon disulfide and many other organic solvents. It forms colorless rhombic crystals that smoke in moist air. It has a monoclinic crystal structure , space group P 2 1 / a (space group no. 14, position 3) , a = 1030.82 pm, b = 710.12 pm, c = 753.64 pm, β = 96.418 °.
Aluminum bromide reacts vigorously with water to form a strongly acidic solution. The Al-Br bonds are largely hydrolyzed here . Aluminum bromide crystallizes from the solution as the hexahydrate AlBr 3 · 6 H 2 O.
use
The main use of aluminum bromide is as a catalyst in organic syntheses such as, for example, polymerization , Friedel-Crafts reactions or bromination .
Individual evidence
- ↑ a b c d e f Entry on aluminum bromide. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
- ↑ a b c data sheet aluminum bromide (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b c d Entry on aluminum bromide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Yoffe, D .; Frim, R .; Ukeles, SD; Dagani, MJ; Barda, HJ; Benya, TJ; Sanders, DC: Bromine Compounds , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2013; doi : 10.1002 / 14356007.a04_405.pub2 .
- ↑ PAETEC formula collection 2003 edition, page 116.
- ^ A b Douglas G. Nicholson et al .: Aluminum bromide . In: Ludwig F. Audrieth (Ed.): Inorganic Syntheses . tape 3 . McGraw-Hill, Inc., 1950, pp. 30-36 (English).
- ↑ RW Berg, FW Poulsen, K. Nielsen: Redetermination of the crystal structure of Al 2 Br 6 . A comparison of three methods . In: Acta Chemica Scandinavica , 1997 , 51 , pp. 442-448. doi : 10.3891 / acta.chem.scand.51-0442 .


